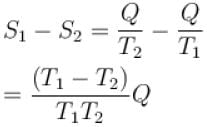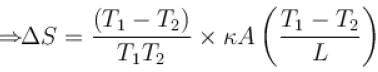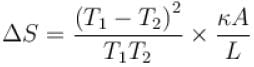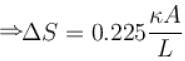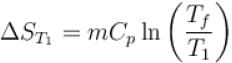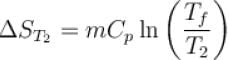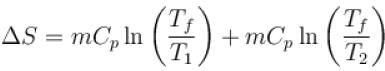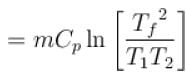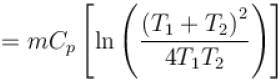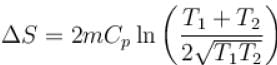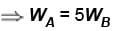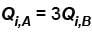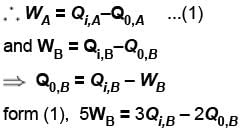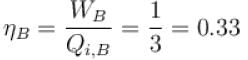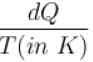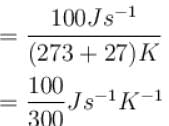Second Law Of Thermodynamics NAT Level - 2 - Physics MCQ
10 Questions MCQ Test - Second Law Of Thermodynamics NAT Level - 2
To make some ice, a freezer extracts 185kJ of heat at –12ºC. The freezer has a coefficient of performance of 5.70. The room temperature is 26ºC. How much heat is delivered to the room (in kJ)
An electric current of 3A flows through a resistance of 10Ω. It is being cooled by running water and is kept at temperature 300K, what is the change in entropy per second of the resistance (in Joules degree).
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Steam at 100ºC is mixed with 1500gms of water at 15ºC so that the final temperature of the mixture is 80ºC the mass of steam is (in gms).
A refrigerator is such that it extracts 20kJ of heat at a lower temperature and give out 50kJ of heat to the surrounding, what is the coefficient of performance (K)
The two ends of a rod of thermal conductivity K, length L and cross-section A are immersed in two heat reservoirs at temp T1 = 800K and T2 = 500K. The rod is thermally insulated from the surroundings. In a steady state, the entropy of the universe in per unit time at the rate of (in units of kA/L)
1000gm of water at temperature 300K is isobarically and adiabatically mixed with an equal mass of water at 500K. Cp = 0.01 cal K-1gm-1 . The entropy change of the universe in (cal K-1 ) is
Engine A, compared to engine B, produces, per cycle, five times the work but receives three times the heat input and exhaust out twice the heat. Determine the efficiency of engine B
At what temperature in Kelvin scale, fluid are assumed to have zero entropy
A mixture of 1.78kg of water and 262g of ice at 0ºC is, in a reversible process, brought to a final equilibrium state where the water/ice ratio, by mass is 1:1 at 0ºC. Calculate the entropy change of the system during this process (in J/K). L = 333 × 103 J/kg for water
An oil bath kept at 27ºC is being supplied heat at the rate of 100Js-1 . Assuming the process to be quasi-static, the rate of increase of entropy of the system is approximately(in JK-1 s-1 )?


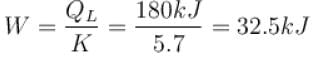

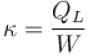
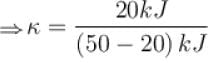
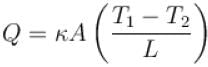 where K , is thermal conductivity.
where K , is thermal conductivity.