Test: General Properties & Compounds of Xenon - NEET MCQ
25 Questions MCQ Test - Test: General Properties & Compounds of Xenon
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
Which of the following is/are correct statement(s) regarding noble gases?
i. He2 molecule does not exist
ii. 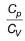 value for noble gases is 1.4
value for noble gases is 1.4
iii. Heaviest noble gas present in air is xenon.
iv. 6th period starts with caesium and ends with radon.
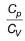 value for noble gases is 1.4
value for noble gases is 1.4iii. Heaviest noble gas present in air is xenon.
iv. 6th period starts with caesium and ends with radon.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The ionisation potential of hydrogen atom is 13.6 eV. Then ionisation potential of He+ ion is
Which of the following is/are correct among the following?
i. XeF2 = sp3d, linear
ii. XeF4 = sp3, square planar
iii. XeO4 = sp3, tetrahedral
iv. XeF6 = sp3d2, octahedral
The oxidation number of oxygen in O2PtF6 is
The number of (pπ-dπ) π-bonds present in XeO3 and XeO4 respectively are
Which of the following reaction occurs?
The wrong statement among the following is
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct
Q.
Which of the following is/are correct regarding noble gases?
Direction (Q. Nos. 16 and 17) This section contains a paragraph, each describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
The noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The lower boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
Direct reaction of xenon with fluorine leads to the formation of a series of compounds with oxidation numbers +2, + 4, + 6 and + 8. XeF4 reacts violently with water to give XeO3. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
Q.
The noble gas compound without lone pair on xenon atom is
The noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The lower boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
Direct reaction of xenon with fluorine leads to the formation of a series of compounds with oxidation numbers +2, + 4, + 6 and + 8. XeF4 reacts violently with water to give XeO3. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
Q.
Which of the following property decreases from helium to xenon?
Direction (Q. Nos. 18 and 19) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Radon is produced upon α-decay of radium atom.
Statement II : α-particles which are equivalent to the atom, reduces the atomic number by 2 and atomic mass by 4 upon decay of mother nucleus.
Statement I : The structure of XeF4 is square pyramidal.
Statement ll : The hybridisation of XeF4 is sp3d2.
Direction (Q. No. 20) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Direction (Q. Nos. 21-25) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
Number of lone pairs on the central atom in XeOF4 are
Among XeF2, XeF4, XeF6, XeO3, XeO4, XeO2, XeOF4 the number of compounds that have non-zero dipole moment.
In XeF2, state the total number of electron pairs in its Lewis dot structure.
In argon, find the number of electrons with spin value + 1/2.
Find the number of unpaired electrons in the fully excited xenon atom.


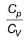 value for noble gases is 1.66
value for noble gases is 1.66














