Revisal Problems (Past 13 Year) JEE Main (P Block Elements) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Year) JEE Main (P Block Elements)
Direction (Q. Nos. 1-30) This section contains 30 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The true statement for 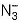 is
is
One mole of each H3PO2, H3PO3 and H3PO4 will neutralise x moles of NaOH. y moles of Ca(OH)2 and z moles of AI(OH)3. Here x, y, z ratio is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When heated which metal nitrate would give the respective metal, oxide NO2 and O2?
Which shape correctly describes the stated molecule?
P4O10 in cold and hot water produce respectively
Among A,B,C, paramagnetic substance(s) is/are
Ammonium dichromate on heating 'X’ gas is liberated. The same gas is liberated by heating
Which has more covalent bonds?
When sulphuric acid reacts with PCI5, sulphuryl chloride is formed as the final product. This shows that sulphuric acid is
S—S bond is present in
I. H2S2O6
II. H2S2O8
III. H2S2O7
IV. H2 S2 O5
Which one of the following cannot act as an oxidising agent?
The V -shape of SO2 is due to the presence
the number of species having two lone pairs of electrons on the central atom according to VSEPR theory is
Liquid oxygen and liquid nitrogen are allowed to flow between the poles of an electromagnet. Choose the correct observation
Among the species, the order of first ionisation energy is
The anion forms an oxyacid of chlorine has the same number of pπ- dπ bonds as that of SO3 (gas), oxidation state of chlorine and the structure of anion is
The products formed when slaked lime reacts with chlorine on heating
Which one among have planar shape ?
Which of the following molecules has a measurable dipole moment?
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
The geometry of the central atom in is
If A represents the central atom, in which molecule F-A-F angle is the smallest?
In the preparation of fluorine, electrolysis of molten KHF2 is preferred than molten fluorspar. The reason is
XeF6 on complete hydrolysis yields ‘X’. The molecular formula of X and its geometry respectively are
The ratio of the heat capacities for one mole of a gas is 1.6. The gas is
Which pair have identical shapes ?
Which classification is correct?
Which of the following is the life saving mixture of asthma patient?
Match the shape to the formula, Which pairing is incorrect?
The amount of Na2S2O3.5H2O required to completely reduce 100 mL of 0.25 N iodine solution is

















