Test: Isomerism in Coordination Compounds - NEET MCQ
25 Questions MCQ Test - Test: Isomerism in Coordination Compounds
Which of the following compounds will show facial and meridional isomerism?
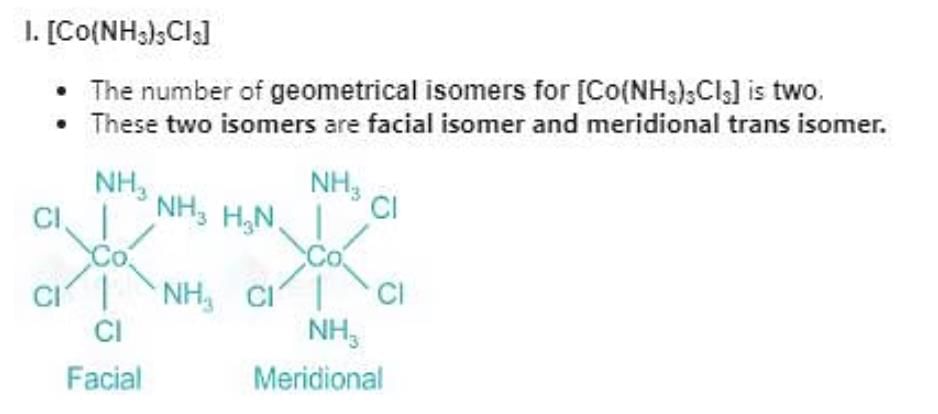
(A)[Co(NH3)3Cl3]
(B)[Co(acac)3]
(C)[Co(dien)(NO2)3]
(D)[Co(gly)3]
(A)[Co(NH3)3Cl3]
(B)[Co(acac)3]
(C)[Co(dien)(NO2)3]
(D)[Co(gly)3]
Which of the following types of octahedral complexes will exhibit geometrical isomerism (where, M = metal, a, b = achiral ligands)?
Which of the following can exhibit linkage isomerism?
What type of isomerism is shown by [Co(NH3)4Br2]Cl?
Which of the following does not show optical isomerism?
The ionisation isomer of [Cr(H2O)4 CI(NO2)]CI is
Which of the following complex species is not expected to exhibit optical isomerism?
The complex [Co(NH3)6[Cr(C2O4)3] and [Cr(NH3)6[Co(C2O4)3] exhibit
Which of the following exhibit cis-trans isomerism?
Which of the following is considered to be an anticancer species?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Linkage isomerism is/are shown by
Which of the following show geometrical isomerism?
Which of the following statements is/are correct?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data etc. Two questions related to the paragraph have been given. Each has only one correct answer among the four given options (a) ,(b), (c) and (d).
Passage
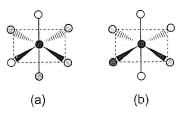
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.
Q.
The oxidation state and coordination number of cobalt in the complex [Co(NH3)4Br2]+ are
Passage
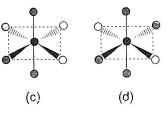
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.


Q.
Which of the structures is identical?
Matching List Type
Direction (Q, Nos. 18 and 19) Choices for the correct combination o f elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
Match the Column ! with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20-24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
Total number of geometrical isomers for the complex [RhCI(CO)(PPh3)(NH3)] is
The total number of isomers of [Co(en)2CI2]+ is
The total number of possible isomers for the complex compound [Cull(NH3)4][PtlICI4]
The number of geometrical isomers possible for Cr(NH3)3CI3 are
For square planar complex of platinum (II), [Pt(NH3)(Br)(CI)py]2+, how many isomeric forms are possible?
Statement Type
Direction ( Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Complexes of MX6 and MX5L type do not show geometrical isomerism. Assume that X and L are unidentate.
Statement Il : Geometrical isomerism is not shown by complexes of coordination number 6.