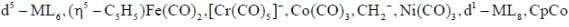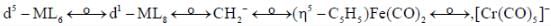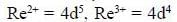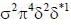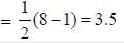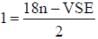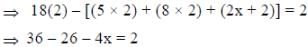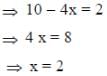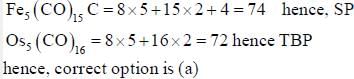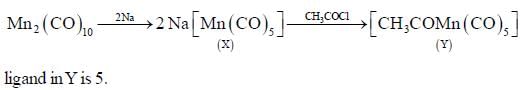Organometallic Compounds - Chemistry MCQ
20 Questions MCQ Test - Organometallic Compounds
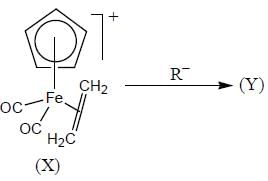
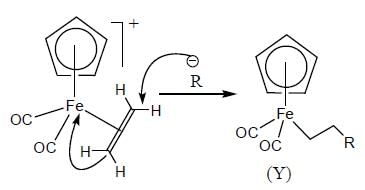
The complex (X) react with nucleophilies (R-) to give stable, neutral metal alkyl complex (Y). The product (Y) is

The 1HNMR spectrum of  at -800C and at 300C are respectively
at -800C and at 300C are respectively
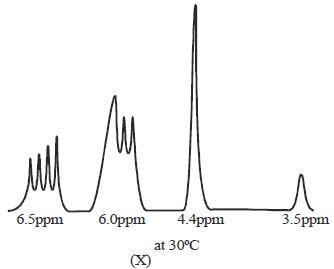
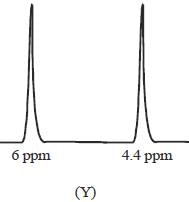
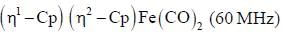 at -800C and at 300C are respectively
at -800C and at 300C are respectively

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The compound 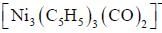 has a single 'CO' stretching absorption at 1761cm-1. The IR data indicate that all C5H5 ligands are pentane to and probably in identical environment. On the basis of these data which of the following statement is true
has a single 'CO' stretching absorption at 1761cm-1. The IR data indicate that all C5H5 ligands are pentane to and probably in identical environment. On the basis of these data which of the following statement is true
Bond lengths and angles have been determined by gas phase electron diffraction for two similar complexes, Fe(CO)4(C2H4)(X) and fe(CO)4(C2F4), (Y)
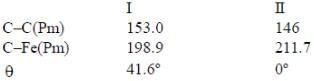
Which of the following statement is true
The solved state magic angle spinning 13C NMR spectrum of Fe(CO)5 shows a single absorption at –26ºC but two singnals at –118ºC with relative intensity
Considering the quadnipolar nature of M -M bond in [Re2CI8]2-, M -M bond order in [Re2Cl4(PMe2Ph)4]+ is ___________
Following the 18e- rule as a guide, determine x in the following complex.
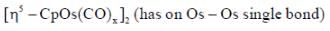
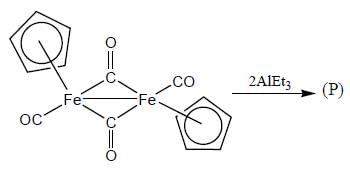
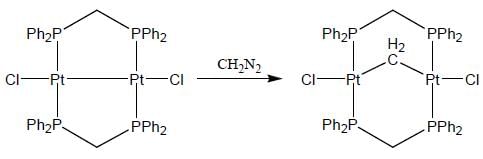
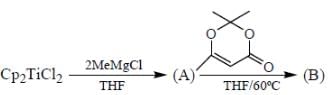
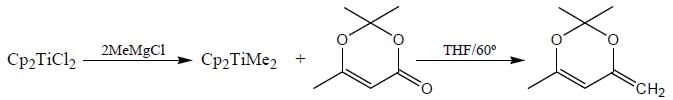
Consider the following reaction, 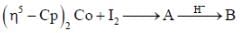
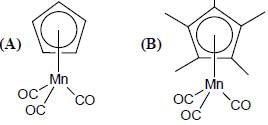
Consider the correct statement regarding A and B
(1) complex A is diamagnetic in nature
(2) complex B contain one ligand in η4-mode
(3) A has smaller M-C bond length than 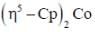
(4) A has larger M-C bond length than 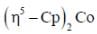
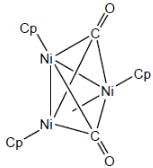
The number of valence electrons provided by Co(CO)4 fragment towards cluster bonding is ___________
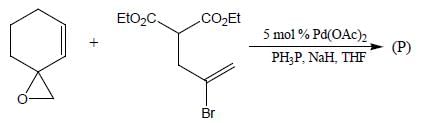
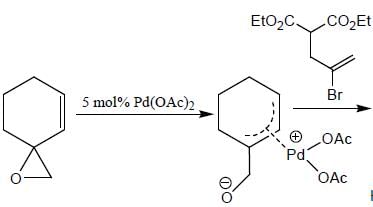
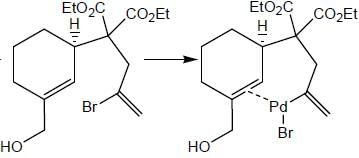
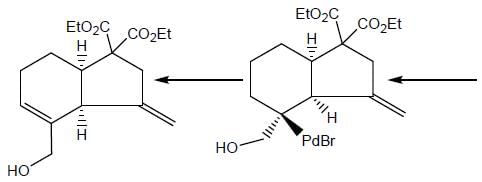
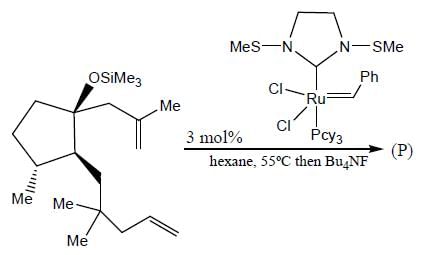
In the reaction shown below
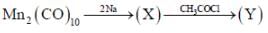
Number of CO ligand in Y is_____


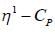 mode looks like
mode looks like 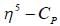 mode. Hence, two signal. At low temperature, this rate is very slow, hence four signal corresponding to 4-types of hydrogen atom. Hence, (X and Y).
mode. Hence, two signal. At low temperature, this rate is very slow, hence four signal corresponding to 4-types of hydrogen atom. Hence, (X and Y).