Quantum Chemistry - 1 - Chemistry MCQ
20 Questions MCQ Test - Quantum Chemistry - 1
The degeneracy of a 3-D S.H.O having energy 9/2hv is _________ (answer should be an integer).
The eigen value of x2e6y with respect to  is _________________ . (answer should be an integer)
is _________________ . (answer should be an integer)
 is _________________ . (answer should be an integer)
is _________________ . (answer should be an integer)| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The number of energy states possible in the range  of a cubic box of side 'a' is _____________ (answer should be an integer).
of a cubic box of side 'a' is _____________ (answer should be an integer).
 of a cubic box of side 'a' is _____________ (answer should be an integer).
of a cubic box of side 'a' is _____________ (answer should be an integer).the angular nodes present in the orbital respected by the wave function  is/are _________________. (answer should be an integer)
is/are _________________. (answer should be an integer)
The correct plot for second excited wave function of a 1-D SHO with the wave function

An electron trapped in a 1-D box of length 1Å having energy 338 eV. This represents
What is the most probable distance from the proton to 1s in a speherical shell. The radial wave solution 
where , Z is the atomic number and a0 is the Bohr radius.
the correct form of decay length in the tunneling through a barrier is
The correct statement (s) is/are
(I) π Bond energy of ethene is 2α
(II) π Bond energy of 1-3-Butadiene is 4.472 β
(III) π Bond energy of benzene is 8β
(Iv) π Bond energy of benzene is 6β
consider a particle of mass 10-30 kg trapped in an infinitely deep potential well of width 0.59 nm. the value of k in ground state is approximately
The wave function for a quantum mechanical particle in a 1-D box of length 'l' is given by 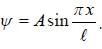 The valur 'A' for a box of length 50 cm is
The valur 'A' for a box of length 50 cm is
The zero point energy for a particle electron in 1-D box of length 1Å in units of n2 eV is ______________ (Round off to one decimal place)
The number of energy levels possible in the range  of a cubic box of side 'a' is _______________ (answer should be an integer)
of a cubic box of side 'a' is _______________ (answer should be an integer)
the minimum energy of a collection of 6 non-interacting electrons of spin -1/2 placed in a one dimensional infinite square well potential of width L is
For a particle of mass m confined in a rectangular box with side 3a and 4a. The energy and degeneracy of third excited state is
The molecular orbital of a  molecule is
molecule is

The electronic charge density on the two hydrogen atoms are equal (0.5). The fround sate wave function  in zero and non-zero overlap approximately is [Given : SAB = 0.4584]
in zero and non-zero overlap approximately is [Given : SAB = 0.4584]
the valance bonding molecule orbital of a hydrogen chloride, molecule may be described as a linear combination of the hydrogen 1s and chlorine 3p atomic orbital 
calculate the probability of finding an electron in a 3p orbital ob chlorine
One molecular orbital of a polar molecule AB has the form  , where ψA and ψB are normalized atomic orbitals centred on A and B, respectively. The electron in this orbital is found on atom B with a probability of 80%. Neglecting the overlap between ψA and ψB , a possible set of cA and cB is
, where ψA and ψB are normalized atomic orbitals centred on A and B, respectively. The electron in this orbital is found on atom B with a probability of 80%. Neglecting the overlap between ψA and ψB , a possible set of cA and cB is





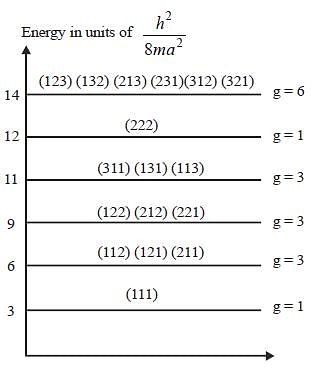








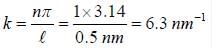
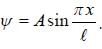 ∵ l= 50
∵ l= 50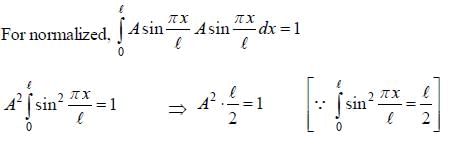
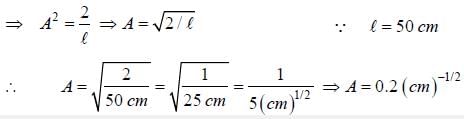
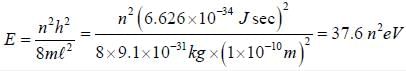

 only two electron with spin 1/2 can fill in the same state
only two electron with spin 1/2 can fill in the same state
























