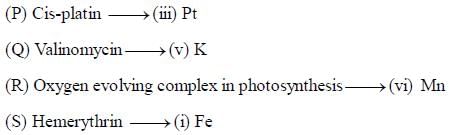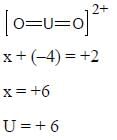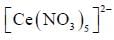Inner Transition Metals & Bio-inorganic - Chemistry MCQ
20 Questions MCQ Test - Inner Transition Metals & Bio-inorganic
The incorrect statement about hemoglobin is:
Which of the following metal is used for magnetic resonance imaging (MRI)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The function and metal associated with carboxy peptidase are respectively:
Which of the following compounds does not involve in the electron transfer in photosynthesis:
The compound which do not involve in the electron transfer in any process of living beings:
Which of the following is not a heme protein?
The substances which act as iron transport and iron storage respectively are:
The transition elements of 4d and 5d series which have no similar size are
The value of spin multiplicity in ground state term of Nd3+ is _____
The value of J in ground state term of Pr3+ is ..........
Which of the following aquated Ln3+ ion is not coloured
The correct statement about Ln3+ ions is
(1) Their magnetic moments increase with increase in number of unpaired electrons
(2) All Ln3+ ions are coloured
(3) The crystal field splitting energy increases with increase in ligand field strength
(4) Most of the Ln3+ ions show sharp bands in their absorption spectra
The lanthanides which show abnormal behaviour towards lanthanide contraction are
The substances containing iron and responsible for oxygen transport are
The correct statement about cytochrome c-oxidase is
The number of oxygen molecules that a molecule hemerythrin can transport is ___________
The most stable oxidation state of Uranium is ........
The coordination number of Ce in 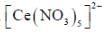 is ______
is ______
Number of metal ion present in active site of Cytochrome C-oxidase is/are_____


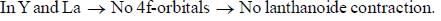
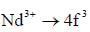
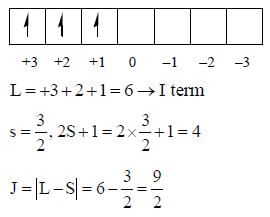
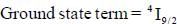
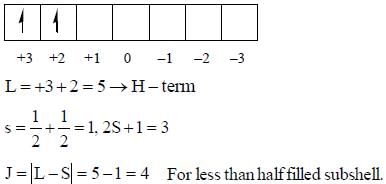
 there is 4f-5d transition. It absorbs in UV. Therefore, it is colourless
there is 4f-5d transition. It absorbs in UV. Therefore, it is colourless