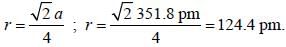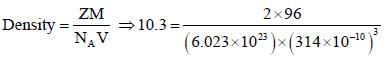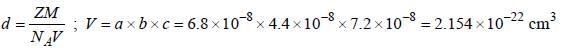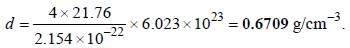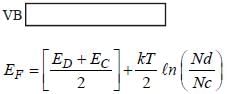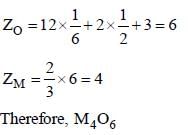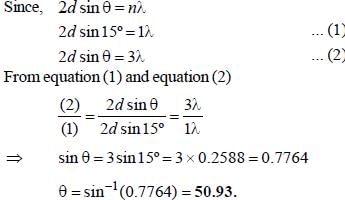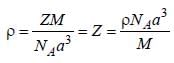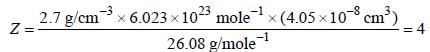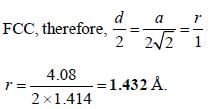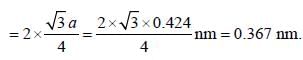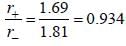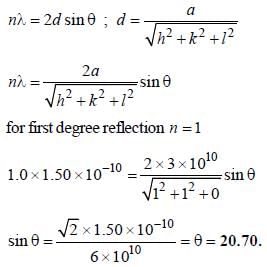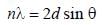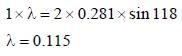Solid State & Group Theory - Chemistry MCQ
20 Questions MCQ Test - Solid State & Group Theory
Nickel forms a face centered cubic unit cell with a = 351.8 pm. The value of crystallographic radius of a nickel atom is
The unit cell of an element of atomic mass 96 and density 10.3 g/cm–3 is a cube with edge length of 314 pm the structure of the crystal lattice is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Lithium boron hydride crystallizes in an orthorhombic system with 4 molecules per unit cell. The unit cell dimensions are 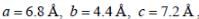 If the molar mass is 21.76. The density of crystal is ______g/cm-3. (Round off to two decimal places).x1
If the molar mass is 21.76. The density of crystal is ______g/cm-3. (Round off to two decimal places).x1
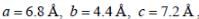 If the molar mass is 21.76. The density of crystal is ______g/cm-3. (Round off to two decimal places).x1
If the molar mass is 21.76. The density of crystal is ______g/cm-3. (Round off to two decimal places).x1A metal crystallizes in simple cubic unit cell. The length of the edge of the unit cell 'a' is 6.22 The radius of each atom of the metal is ______(
The radius of each atom of the metal is ______( ) (Round of to two decimal places).
) (Round of to two decimal places).
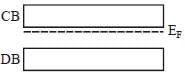
In n-type semi conductors the correct statement is/are
(i) The fermi energy level lies in between donor band and valence band
(ii) The fermi energy level lies in donor band and conduction band
(iii) The fermi energy increases with increasing temperature
(iv) The fermi energy decreases with increasing temperature
In an ionic oxide, oxide ions are arranged in hcp array and positive ion occupy two thirds of octahedral void simplest formula of metal M is
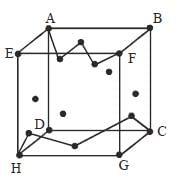
In case of Fullerene the correct relation between number of faces (F), number of vertices (V) and number of edges (E) is
The first order reflection of a beam of X-rays from a given crystal occur at 15o. The 3rd order reflection will occurs at angle of _____ degree. (Round off to two decimal places).
A metal (atomic weight = 26.08), crystallizes in the cubic system with 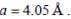 Its density is 2.7g/cm-3. The radius of the atom of this metal will be _____
Its density is 2.7g/cm-3. The radius of the atom of this metal will be _____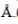 (Round off to two decimal places).
(Round off to two decimal places).
The distance between the body-centered atom and one corner atom in sodium a =0.424 nm is ____nm. (Round off to two decimal places).
The ionic radii of Cs+ and Cl-1 ions are 1.69 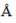 and 1.81
and 1.81 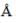 respectively. The co-ordinate number of Cs+ is _____(answer should be an integer).
respectively. The co-ordinate number of Cs+ is _____(answer should be an integer).
For primitive cubic crystal with a = 3 x 1010m, the smallest diffraction angle θ, for (110) plane for 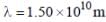 is ______(Round off to two decimal places).
is ______(Round off to two decimal places).
The first order diffraction of X-rays from a ceratin set of crystal planes occurs at an angle of 11.8º from the planes, if the planes are 0.281 nm a part the wavelength of X-rays is
Match the crystal system unit cells mentioned in column-I with their characteristic feature mentioned in column-II

The Mulliken Notation for the following irreducible representation
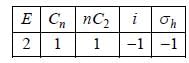
The number of classes in CH4 molecule is/are ________(answer should be an integer).