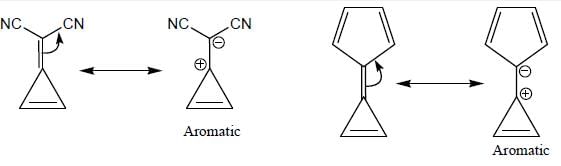
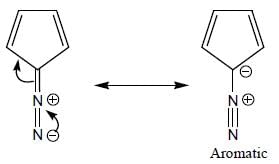
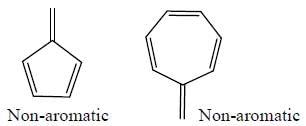
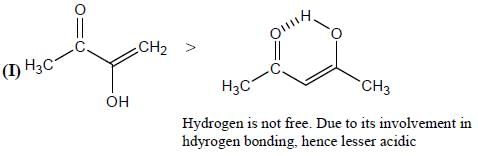
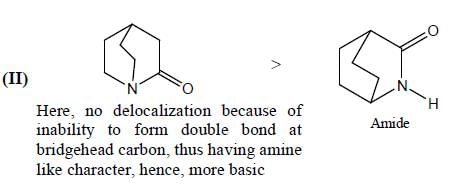
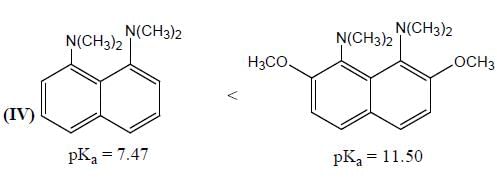
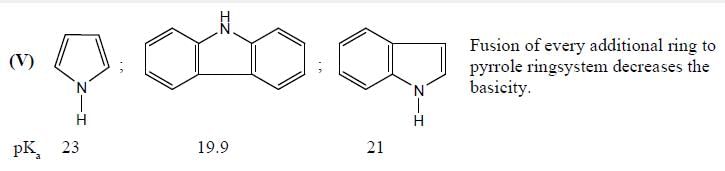
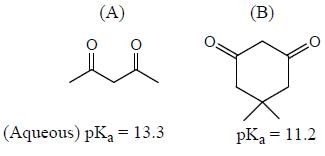
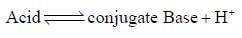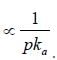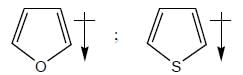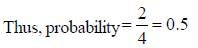GOC & Aromaticity - 1 - Chemistry MCQ
20 Questions MCQ Test - GOC & Aromaticity - 1
Which is more nucleophilic than the other of the following compounds?
P. (X) R2N- (Y) R-O- (Z) F-
Q. (X) R-S- (Y) Cl-
R. (X) R-S- (Y) R-O-
S. (X) R-OH (Y) R-SH
T. (X) OH- (Y) -O-O-H
U. (X) NH3 (Y) H2N-NH2
P. (X) R2N- (Y) R-O- (Z) F-
Q. (X) R-S- (Y) Cl-
R. (X) R-S- (Y) R-O-
S. (X) R-OH (Y) R-SH
T. (X) OH- (Y) -O-O-H
U. (X) NH3 (Y) H2N-NH2
The correct order of the pKa values for the conjugate acids of heterocyclic compounds given below is:

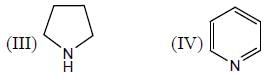

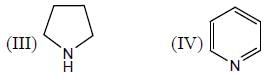
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
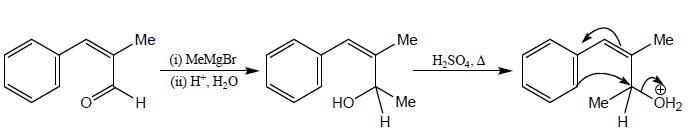
Consider the following reaction sequence:
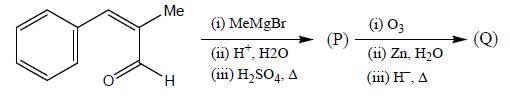
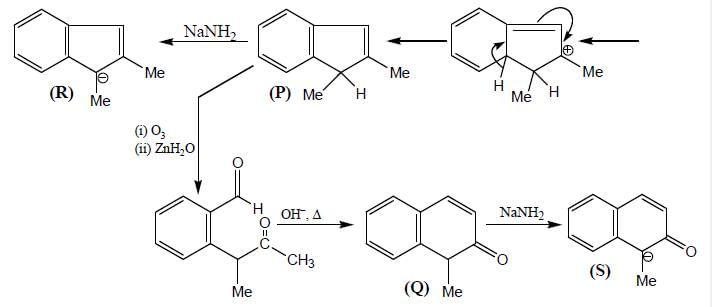
Product (P) and (Q) are made to react with sodium amide to yield R and S respectively. Identify the correct statement.
Consider the bonds marked as (A), (B), (C) and (D) in the following structures.
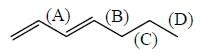
What is the correct order of their homolytic bond dissociation energies?
The correct order of dipole moment in pyrrole, thiophene and furan respectively, is:
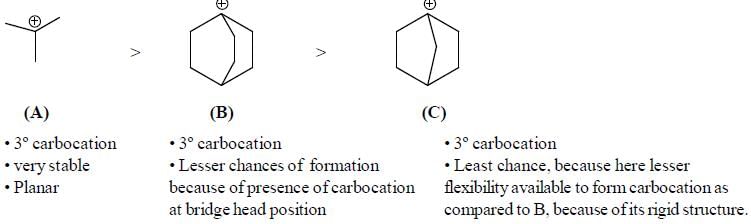
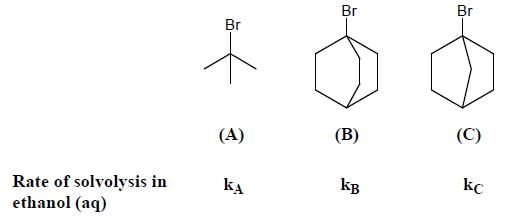
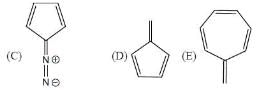
The rotational energy barrier along 1-2 bond will be the lowest in:
Consider the following reactions,
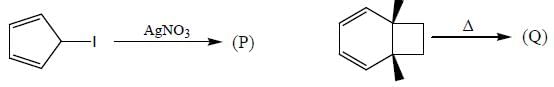
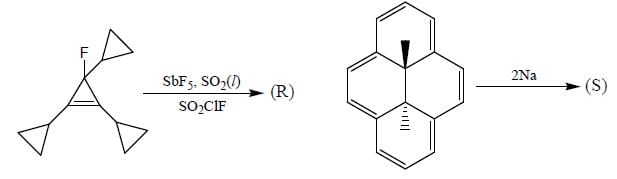
A boy is said to choose one entity from product P, Q, R and S. What is the probability that the chosen compound will be antiaromatic? (Answer should be in decimals)
Given compounds A,B,C and D are subjected to IR spectrum analysis. compounds which will show minimum and maximum vco streching frequency in IR respectively will be:

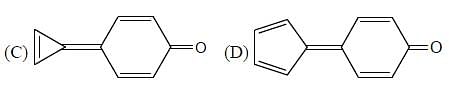
If polarity of solvent is sufficiently increased, then what will happen?
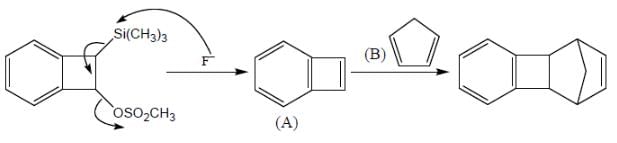
Consider the following sequence of reaction,
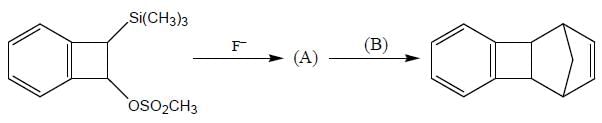
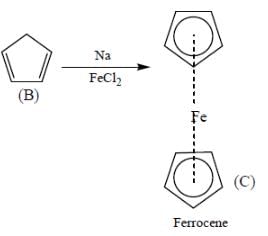
B on reaction with Na in presence of FeCl2 gives a well known organometallic compound C. Identify the correct statement about above reaction sequence.
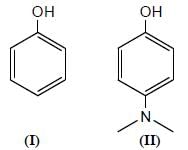
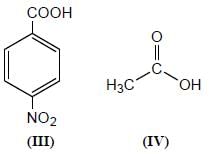
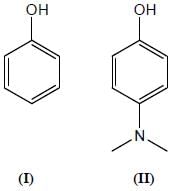
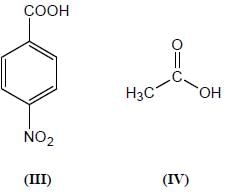
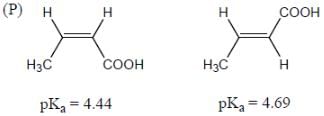
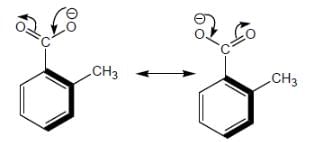
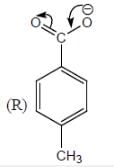
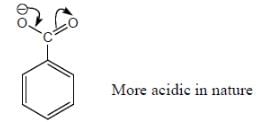
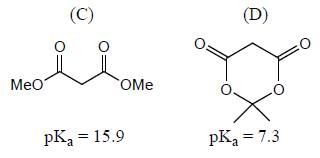
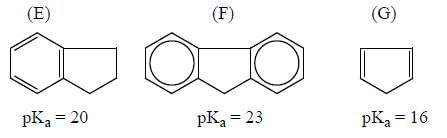
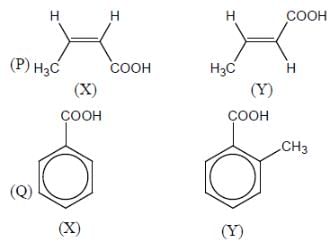
Among the pairs given below, identify the most acidic compound.
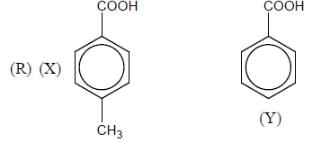
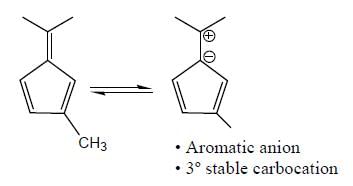
The number of anti-aromatic systems present among the following compounds is/are _____________(Answer should be an integer)




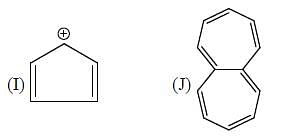
Among the following, how many are aromatic in nature? (Answer should be an integer)
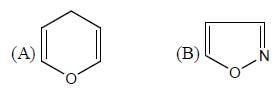
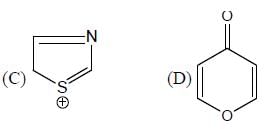
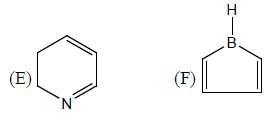
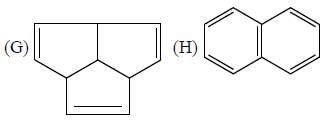
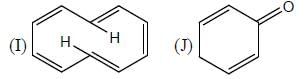
The number of aromatic compound among the following will be: (Answer should be an integer)
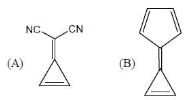
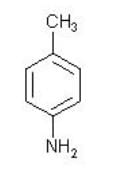
What is the sequence of reagents that will accomplish the synthesis of the following aromatic amine from benzene?
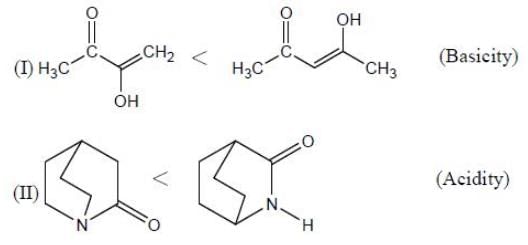
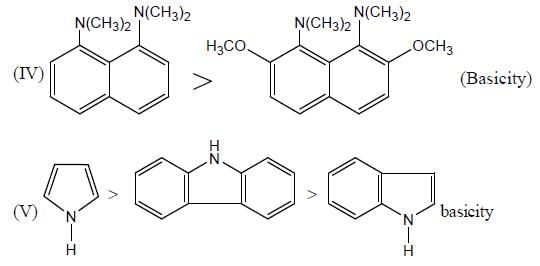
In the following compounds, how many pairs of compounds having correct order w.r.t mentioned properties? (Answer should be an integer)
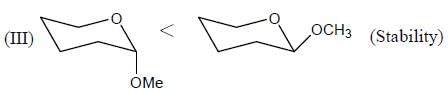
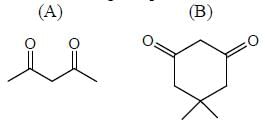
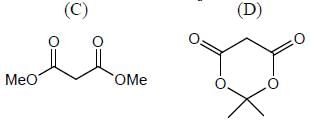
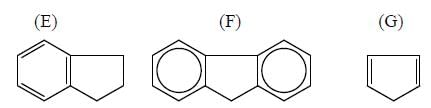
In the following compounds, how many compounds having more pKa value relative to the H2O? (Answer should be an integer)


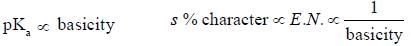
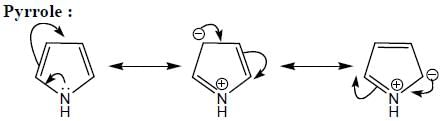
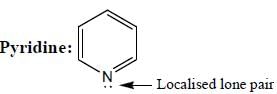
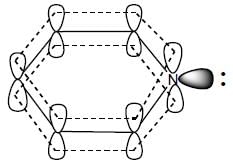
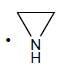 (Aziridine having more s% character than pyrrolidine. so, pyrrolidine is more basic than Aziridine).
(Aziridine having more s% character than pyrrolidine. so, pyrrolidine is more basic than Aziridine).