Test: E2 Reaction Advanced, E1 Cb & Hofmann Elimination - NEET MCQ
20 Questions MCQ Test - Test: E2 Reaction Advanced, E1 Cb & Hofmann Elimination
Only One Option Correct Type
Direction (Q. Nos. 1-5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
What is the major alkene product in the following reaction?
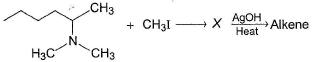
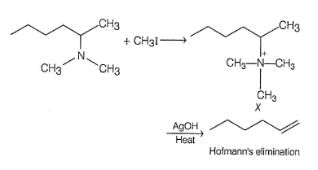
Which of the following gives 1-butene as the major product most easily on heating with AgOH?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
1-butene would be formed most easily in the following reaction when X is
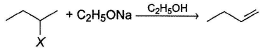
Consider the following reaction and the product formed.
Q.
The most likely mechanism of the above reaction is
One or More than One Options Correct Type
Direction (Q. Nos. 6-8) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
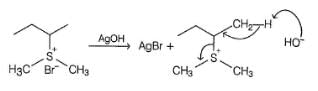
Which of the following gives methanol as the major product when heated with AgOH?
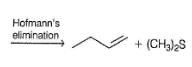
Which of the following gives cis-2,3-diphenyl, 1-2-butene as the major E2 product, when treated with ethanolic KOH solution?
The following elimination reaction, if proceeds by E1cb mechanism, equilibrium mixture would consist of
Comprehension Type
Direction (Q. Nos. 8-16) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Nine questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
The following E2 is carried out with different halogen substituent:
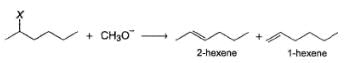

From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
Based to the above observation, which flask will have the largest amount of 1-hexene?
Passage I
The following E2 is carried out with different halogen substituent:


From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
Highest percentage yield of 1-hexene in flask 4 leads us to conclude that
Passage I
The following E2 is carried out with different halogen substituent:


From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
During formation of products in the given elimination reaction, the highest energy transition state would have been produced when methoxide ion reacts with
Passage I
The following E2 is carried out with different halogen substituent:
From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
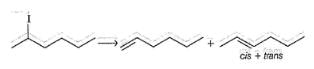
If we consider X to be iodine in the given elimination reaction, the number of different elimination products present in the flask-1 would be?
Passage I
The following E2 is carried out with different halogen substituent:


From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
Transition state with maximum double bond character will be formed when methoxide reacts with
Passage I
The following E2 is carried out with different halogen substituent:


From the percentage yield of two products observed with different leaving group, it was concluded that both ease of leaving group and steric hindrance to the approach of base CH3O- to β-H affect the orientation of elimination reaction.
Q.
Transition state with least double bond character will be formed when methoxide reacts with
Passage II
When compound 1 is heated with C2H5ONa compound 2 and 3 are formed:
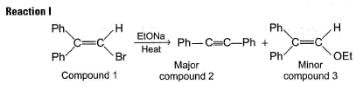
Two mechanisms were proposed for reaction I.
Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.
Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2
To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.
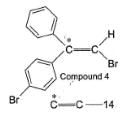
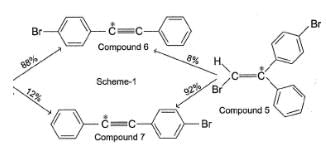
Q.
Based on the results of scheme 1, the chemist most likely ruled out mechanism A because they assumed that intermediate A should have formed
Passage II
When compound 1 is heated with C2H5ONa compound 2 and 3 are formed:

Two mechanisms were proposed for reaction I.
Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.
Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2
To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.


Q.
Compound 2 and 6 can be distinguished from each other by all of the following techniques except:
Passage II
When compound 1 is heated with C2H5ONa compound 2 and 3 are formed:

Two mechanisms were proposed for reaction I.
Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.
Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2
To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.


Q.
In reaction scheme 1, had the α-C to bromine be labelled with C-14
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination o f elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
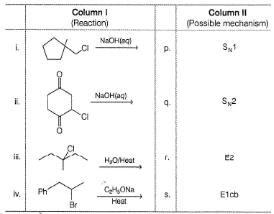
Q.
Match the reaction from Column I with the type of m echanism from Column II and mark the correct option from the codes given below.
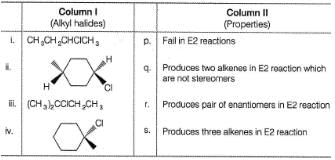
Match the alkyl halides in the Column I with the properties of their products produced in E2 elimination reaction in Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20 and 21) This section contains 2 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
In the reaction given below how many elim ination products are formed in principle if reaction proceeds by E1 cb mechanism?
Consider the following reaction,
Q.
How many different stereoisomers of the major elimination product Y are possible?