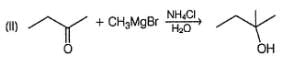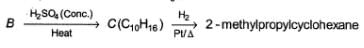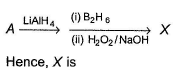Test: Preparation of Alcohols - NEET MCQ
22 Questions MCQ Test - Test: Preparation of Alcohols
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q,
Which of the following pairs of compounds can be used as starting material in the synthesis of 2-phenyl-2-pentanol?
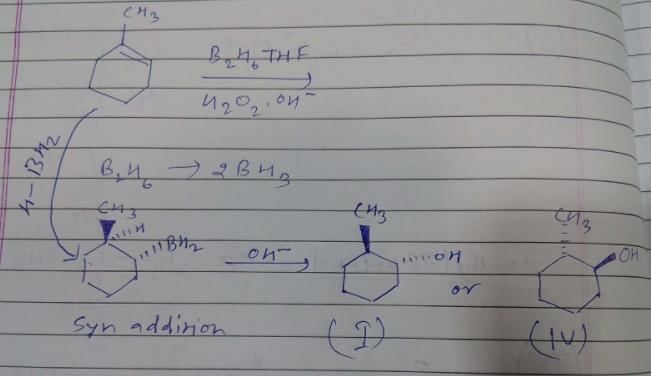
What is the product of the reaction of methyl cyclohexene with B2H6 in THF followed by the oxidation with alkaline H2O2?



| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
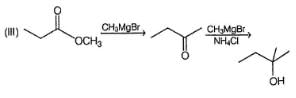
Choose the reagent and reactant that would produce 2-methyl-2-butanol as the major product.
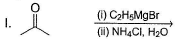
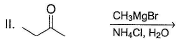
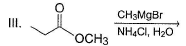
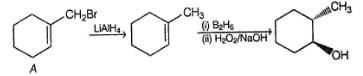
What is the correct structure for the major compound produced by the following reaction sequence?
In the reaction given below,
The final major organic product is
Primary alcohol can easily be prepared from primary alkyl halide via SN2 reaction with aqueous NaOH. However, similar method does not work for the preparation of tertiary alcohol. Which reaction can be used for the efficient preparation of tertiary alcohol {tertiary butanol) from tertiary butyl bromide?
What is the major product in the following reaction?
Direction (Q. Nos. 9-15) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
In the following reaction,
The possible substitution product(s) is/are
In the following reaction,
Possible product(s) is/are
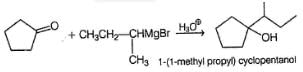
Which of the following Grignard’s synthesis can result into 2-cyclopentyl-2-butanol?
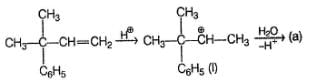
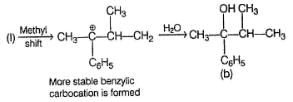
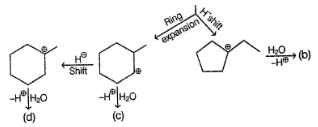
Aliphatic alcohol when treated with dilute H2SO4, undergo isomerisation via reversible reaction. In the following reaction, which of the isomers are expected to be present at equilibrium?
In the following reaction,
The alcohol(s) formed in significant yield is/are
In the reaction given below,
The correct statement regarding the outcome of the above reaction is/are
Consider the following reaction,
Possible product(s) is/are
Comprehension Type
Direction (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following sequence of reaction,
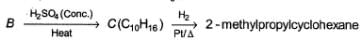
Q.
The structure of compound B is
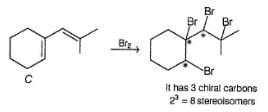
Consider the following sequence of reaction,
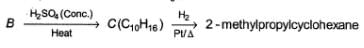
Q.
If C is treated with excess of Br2(l) how many different isomers of bromination product(s) result?
One Integer Value Correct Type
Direction (Q. Nos. 19-22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
In the following reaction,
Q.
How many different diols are formed as a result of nucleophilic addition reaction?
In the reaction given below,
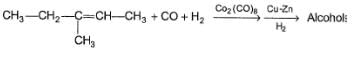
How many different products are expected?
If 3, 3-dimethyl-2, 4-pentanedione is treated with a Grignard reagent consisting of mixture of CH3MgBr and C2H5MgBr and finally hydrolysing product with dilute H2SO4 results in the formation of how many different diols?
If a pure enantiomer of 3-methyl-1-pentene is treated with boiling solution of dilute H2SO4, how many different alcohols are expected in principle?