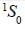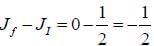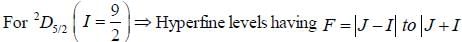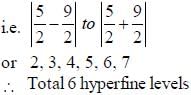Test: Atomic & Molecular Physics - 2 - Physics MCQ
20 Questions MCQ Test - Test: Atomic & Molecular Physics - 2
The rotational lines seperation in Raman experiment for HC1 molecule is 41.6 cm-1 The inter nuclear distance is________Å
The rotational inertia for HI molecule is 4.5 x 10-48 kg m2. The temperature at which the average translational kinetic energy of molecule equals to difference of ground rotational static and First excited state is___________K.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Total number of vibrational degree of freedom of H2 O2 is__________________
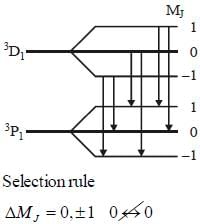
The number of Zeeman lines of the spectrum line 3D1 → 3P1 in presence of weak magnetic field is______________
For an atom in the state 2D5/2 the lande-g-factor should be__________
Let E1, E2, E3, be the first three energy levels of H-atom. Consider the ratio (E3—E1)/(E2-E1). Neglecting the fine structure condition, this ratio is approximately____________
Two monochromatic sources, L1 and L2 emit light at 600 nm and 700 nm respectively. If their frequency band width are 10-1 and 10-3 GHz respectively, then ratio of line width L1 and L2 is approximately___________
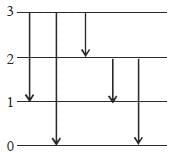
The figure (shown) depicts the energy levels of a 4 level atomic system with the Einstein A coefficients as follows
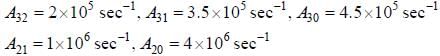
The life time of the atomic level 3 is
CO2 molecule lias the first tew energy levels uniformly seperatedby approximately 2.5 meV at a tempera tine of300 K. the ratio of the number of molecules in the 4th excited state to the number in 2nd excited state is about
The ground state terms for nd10, np4, np3 and nd8 are respectively in L-S coupling are
If the wavelength of the first line of Lyman series of hydrogen is 1215 Å. the wavelength of the second line of the series is
The Kβ line of X-ray emitted from an atom with principal quantum number n = 1, 2, 3, arise from the transition.
If the line taken by an electron to traverse the first orbit in hydrogen atom is T. then the time taken by the electron to traverse the second orbit in lithium atom (Li++) is
If the atom is in the 3D3 state, the angle between it’s orbital and spin angular momentum vectors is
Which of the following transition are allowed
Sodium atom ground state configuration is 1s2 2s2 2P6 3s1. by emitting 1e- Na changes to Na+ having configuration 1s2 2s2 2P6 in the transition the change in J value is
According to the electric dipole transition, which is correct
Nuclear spin 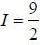 for bismuth atom The number of levels in which the term
for bismuth atom The number of levels in which the term 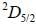 of bismuth will split up?
of bismuth will split up?
For pure rotatioal Raman spectrum of a linear diatomic molecule is recorded using Electromagnetic radiation of frequency v6. The frequency of the two consective stokes lines are


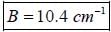
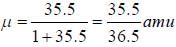
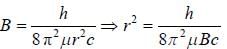
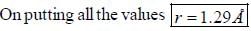
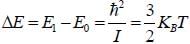
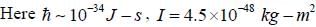
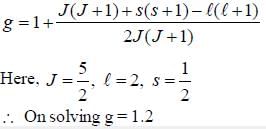
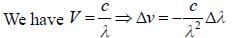
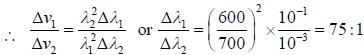
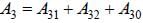
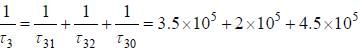
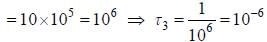
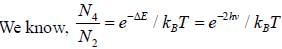
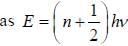
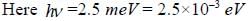
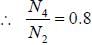
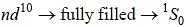
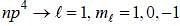
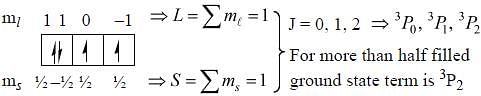
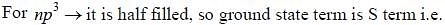
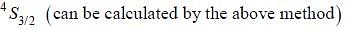
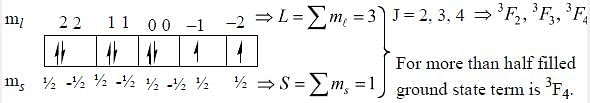
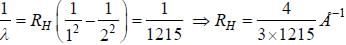
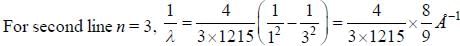
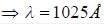
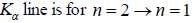
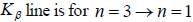
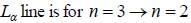
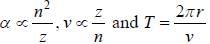
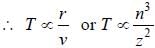
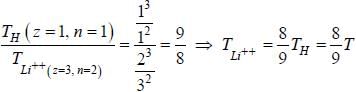
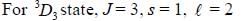
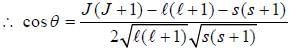
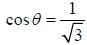

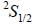 while the new state Na+ has ground term
while the new state Na+ has ground term