Revisal Problems (Past 13 Year) JEE Main (Aliphatic Aldehydes And Ketones) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Year) JEE Main (Aliphatic Aldehydes And Ketones)
Only One Option Correct Type
Direction (Q. Nos. 1-30) This section contains 30 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
What is the IUPAC name of the following compound?
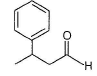
What is the major organic product obtained from the following reaction?
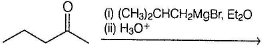
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the major organic product obtained from the following reaction?
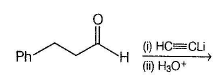
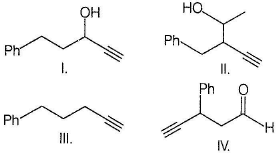
What is the major organic product obtained from the following reaction ?
Which ketone-diol undergoes cyclisation to form the following acetal?
What is the major organic product obtained from the following reaction?
What is the major organic product obtained from the following reaction?
What is the major organic product obtained from the following sequence of reaction?
What is the major organic product obtained from the following sequence of reaction?
Which of the following has the highest Keq for enolisation?
Which of the following is the best description of the mechanism of the reaction between a ketone and an amine to form an imine?
Which of the following statement is not true?
Which of the following is a reactive intermediate in the reaction of acetone with bromine in acetic acid to form 1 -bromo-2-propanone?
Which of the following reactions can be used to prepare the compound given below?
Compound which does not give stereoisomers on treatment with hydroxylmine is
The most stable enol from of the compund
What is the major product in the following reaction?
Give the product of the following reaction.
Which of the following reagent could be used to differentiate the following compounds by a visible change? 2-butanone and propanoic acid
An organic compound X produces Y in the first step of oxidation which on further oxidation gave an acid. Y on treatment with ammoniacal silver nitrate solution gave a black precipitate. X is likely to be a
The major product of the foilowing reaction is
Which represents the best method for converting a carboxylic acid to an aldehyde?
You have two C6H10O ketones, I and II. Both are optically active, but I is racemised by treatment with base and II is not. Wolff-Kishner reduction of both ketones give the same achiral hydrocarbon, formula C6H12. What reasonable structures may be assigned to I and II?
A C5H12O compound is optically active, and is oxidised by PCC in CH2CI2 to an optically active C5H10O product, which is racemised in acid or base. Which of the following best fits these facts?
Which of the following compounds would not be a possible product from the mixed aldol reaction of acetaldehyde and butanal?
Which reaction or sequence of reactions would be best used to convert cyclohexanone to cis-1,2-cyclohexanediol?
Arrange the following in the increasing order of reactivity towards aldol condensation reaction
Provide the most suitable set of reagents required for the following trans formation.
The major organic products in the following reaction are
I. HCOOH
II. C6H5COOH
III. CH3OH
IV. C6H5CH2OH
The major organic product formed in the following reaction is

















