CPU 5 - Esters (Carboxylic Acids And Acid Derivatives) - Class 12 MCQ
25 Questions MCQ Test - CPU 5 - Esters (Carboxylic Acids And Acid Derivatives)
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
(+) -2-butanol is first treated with tosyl chloride then with C6H5ONa There was obtained secondary butyl benzoate. This ester when subjected to alkaline hydrolysis, alcohol and acid are recovered back. What is true about stereochemistry of 2-butanol obtained after hydrolysis?
Predict the major addition product of the following reaction
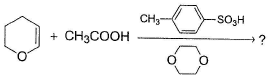
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the major organic product of the following reaction?
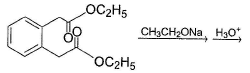
Choose the best method that could perform the following transformation.
Arrange the following esters in the increasing order of reactivity in base catalysed hydrolysis reaction.
Which ester given below can most easily be produced by acid catalysed esterification (Fischer’s esterification)?
Which of the following ester is most likely to undergo unimolecular acid catalysed hydrolysis reaction?
Which reaction and indicated product with specified stereochem istry is most accurate?
One or More than One Options Correct Type
Direction (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
What is/are true regarding the following Fischer’s ester synthesis?
Consider the following acid catalysed esterification reaction,
Q.
The correct statement concerning the above reaction is/are
In which of the following hydrolysis reaction of esters, reactant and products are correctly matcheed ?
In which of the following reaction(s), reactant and products are correctly matched?
If is treated with
in the presence of H2SO4, possible esters formed is/are
The correct statement regarding the following transformation is/are
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Treatment of 2,4-pentanedione with excess of KCN(aq)and acetic acid followed by hydrolysis in the presence of H2SO4 gives two products A and B, both having molecular formula C7H12O6. When heated, B first gives a lactonic acid C7N10O5 and finally a dilactone P (C7H8O4).
Q.
What is the correct structure of A?
Treatment of 2,4-pentanedione with excess of KCN(aq)and acetic acid followed by hydrolysis in the presence of H2SO4 gives two products A and B, both having molecular formula C7H12O6. When heated, B first gives a lactonic acid C7N10O5 and finally a dilactone P (C7H8O4).
Q.
What is the correct structure of P?
Treatment of 2,4-pentanedione with excess of KCN(aq)and acetic acid followed by hydrolysis in the presence of H2SO4 gives two products A and B, both having molecular formula C7H12O6. When heated, B first gives a lactonic acid C7N10O5 and finally a dilactone P (C7H8O4).
Q.
A also lactonises on heating. What is the correct structure of lactone obtained from A?
Statement Type
Direction (Q. Nos. 18-21) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Ester formation from acid and alcohol occur in acidic medium but not in alkaline medium. However, hydrolysis of esters proceeds in both acidic and alkaline medium.
Statement II : In alkaline medium carboxylic acid is neutralised into salt which do not undergo nucleophilic attack by alcohols.
Statement I : When ethyl propanoate is refluxed with butanol in slightly acidic condition at the boiling point of ethanol, butylpropanoate is formed.
Statement II : During trans esterification, larger alcohols always displaces the smaller one.
Statement I : If CH3COOCH(CH3)C6H5 is heated with dilute NaOH solution, racemic mixture of 1-phenyl ethanol is formed.
Statement II : At low hydroxide concentration and alcohol part of ester being capable of forming stable carbocation, hydrolysis proceeds preferably by unimolecular alkyl oxygen cleavage mechanism.
Statement I : p-nitrobenzoic acid is more reactive than benzoic acid in acid catalysed esterification reaction.
Statement II : Rate determining step in Fischer’s esterification reaction of carboxylic acid in nucleophilic attack alcohols on protonated acid.
One Integer Value Correct Type
Direction (Q. Nos. 22-25) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
If a mixture containing ethyl acetate and ethyl propanoate is heated in the presence of C2H5ONa, how many different condensation products would be formed?
In the following lists of reactions, how many of them gives ester as the major organic product?
How many different isomers exist for C3H6O2 which reduces Tollen’s reagent as well as forms C5H8O3 upon treatment with acetic anhydride ?
A hydroxy acid has molecular formula C5H10O3 and it iactonises (forms cyclic esters) on heating. If only five and six membered lactones are considered, how many different isomers of lactones are possible?

















