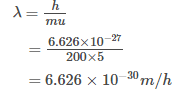MCQ(Previous Year Questions) - Structure Of Atom (Level 2) - JEE MCQ
18 Questions MCQ Test - MCQ(Previous Year Questions) - Structure Of Atom (Level 2)
The energy of an electron in the first Bohr orbit of H atom is -13.6 eV. The possible energy value(s) of the excited state(s) for electrons in Bohr orbits of hydrogen is/are : [JEE 1998]
Which of the following statement(s) is (are) correct ? [JEE 1998]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Assertion: Zn2+ is diamagnetic. [JEE 1998]
Reason: The electrons are lost from 4s orbital to form Zn2+.
The electrons, identified by quantum numbers n and l
(i) n = 4, l = 1,
(ii) n = 4, l = 0
(iii) n = 3, l = 2 and
(iv) n = 3, l = 1
can be placed in order of increasing energy, from the lowest highest, as [JEE 1999]
Electronic configuration of an element is 1s22s22p63s 23p63d54s1. This represents its [JEE 2000]
The wavelength associated with a golf ball weighing 200 g and moving at a speed of 5 m/h is of the order
[JEE 2001]
The number of nodal planes in a px orbital is : [JEE 2000]
The quantum numbers +1/2 and -1/2 for the electron spin represent : [JEE 2001]
Rutherfords experiment, which established the nuclear model of atom, used a beam of : [JEE 2002]
The radius of which of the following orbit is same as that of the first Bohr's orbit of hydrogen atom? [JEE 2004]
The number of radial nodes of 3s and 2p orbitals are respectively. [JEE 2004]
Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecular B2 is [JEE 2010]
Ground state electronic configuration of nitrogen atom can be represented by [JEE 1999]
The hydrogen-like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom. [JEE 2010]
Q. The state S1 is
The hydrogen-like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom. [JEE 2010]
Q. Energy of the state S1 in units of the hydrogen atom ground state energy is
The hydrogen-like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom. [JEE 2010]
Q. The orbital angular momentum quantum number of the state S2 is
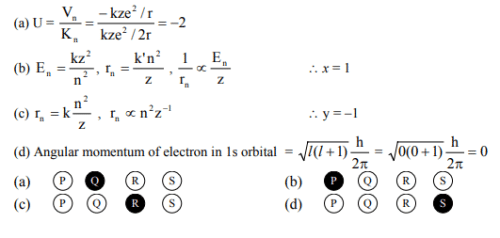
Given in hydrogenic atom rn, Vn, E, Kn stand for radius, potential energy, total energy and kinetic energy in nth orbit. Find the value of U, v, x, y. [JEE 2006]
Match the entries is Column-I with the correctly related quantum number(s) in Column-II. Indicate your answer by darkening the appropriate bubbles of the 4 × 4 matrix given in the ORS. [JEE 2008]