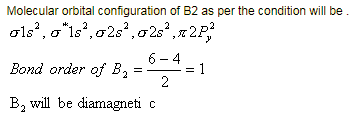MCQ (Previous Year Questions) - Chemical Bonding & Molecular Structure (Level 2) - JEE MCQ
25 Questions MCQ Test - MCQ (Previous Year Questions) - Chemical Bonding & Molecular Structure (Level 2)
Specify hybridization of N and B atoms in a 1 : 1 complex of BF3 and NH3 [JEE 2002]
The nodal plane in the p-bond of ethene is located in [JEE 2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Identify the least stable ion amongst the following [JEE 2002]
Which of the following molecular species has unpaired electron (s) ? [JEE 2002]
Which of the following are isoelectronic and isostructural ? NO3_, CO32_, ClO3_, SO3 [JEE 2003]
According to molecular orbital theory which of the following statement about the magnetic character and bond order is correct regarding O2+ [JEE 2004]
Which species has the maximum number of lone pair of electrons on the central atom ? [JEE 2005]
The percentage of p-character is the orbitals forming P_P bonds in P4 is [JEE 2007]
Among the following, the paramagnetic compound is [JEE 2007]
The species having bond order different from that in CO is [JEE 2007]
The structure of XeO3 is [JEE 2007]
Statement-1 : p-Hydroxybenzoic acid has a lower boiling point than o-hydroxybenzoic acid.
Statement-2 : o-Hydroxybenzoic acid has intramolecular hydrogen bonding. [JEE 2007]
Statement-1 : In water, orthoboric acid behaves as a weak monobasic acid.
Statement-2 : In water, orthoboric acid acts as a proton donor. [JEE 2007]
Statement-1 : Pb4+ compounds are stronger oxidizing agents than Sn4+compounds. [JEE 2008]
Statement-2 : The higher oxidation states of group 14 elements are more stable for the heavier members of the group due to 'inert pair effect'.
Match each of the diatomic molecules in Column - I with its property/ properties in Column-II.
Column-I Column-II [JEE 2009]
(A) B2 (P) Paramagnetic
(B) N2 (Q) Undergoes oxidation
(C) O2— (R) Undergoes reduction
(D) O2 (S) Bond order ³ 2
(T) Mixing of 's' and 'p' orbitals
The nitrogen oxide(s) that contain(s) N_N bond(s) is (are). [JEE 2009]
In the reaction [JEE 2009]
2X + B2H6 ® [BH2(X)2]+[BH 4]_ the amine(s) X is (are) :
The species having pyramidal shape is [JEE 2010]
Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is [JEE 2010]
All the compounds listed in Column I react with water. Match the result of the respective reactions with the appropriate options listed in Column II.
Column-I Column-II [JEE 2010]
(A) (CH3)2SiCl2 (P) Hydrogen halide formation
(B) XeF4 (Q) Redox reaction
(C) Cl2 (R) Reacts with glass
(D) VCl5 (S) Polymerization
(T) O2 formation
In Allen (C3H4), the type (s) of hybridisation of the carbon atoms is (are) [JEE 2012]
The shape of XeO2F2 molecule is [JEE 2012]
With respect to graphite and diamond, which of the statement(s) given below is (are) correct?
Decreasing order of the O_O bond length present in them O2, KO2 and O2[AsF4] [JEE 2004]
The value of n in the molecular formula BenAl2Si6O18 is [JEE 2010]