Class 10 Exam > Class 10 Tests > Test :- Chemical Reactions And Equations Class –10 - Class 10 MCQ
Test :- Chemical Reactions And Equations Class –10 - Class 10 MCQ
Test Description
10 Questions MCQ Test - Test :- Chemical Reactions And Equations Class –10
Test :- Chemical Reactions And Equations Class –10 for Class 10 2024 is part of Class 10 preparation. The Test :- Chemical Reactions And Equations Class –10 questions and answers have been prepared
according to the Class 10 exam syllabus.The Test :- Chemical Reactions And Equations Class –10 MCQs are made for Class 10 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test :- Chemical Reactions And Equations Class –10 below.
Solutions of Test :- Chemical Reactions And Equations Class –10 questions in English are available as part of our course for Class 10 & Test :- Chemical Reactions And Equations Class –10 solutions in
Hindi for Class 10 course.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free. Attempt Test :- Chemical Reactions And Equations Class –10 | 10 questions in 5 minutes | Mock test for Class 10 preparation | Free important questions MCQ to study for Class 10 Exam | Download free PDF with solutions
Test :- Chemical Reactions And Equations Class –10 - Question 1
Which of the following is not a chemical reaction?
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 1
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test :- Chemical Reactions And Equations Class –10 - Question 3
The galvanisation of iron means coating iron with :
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 3
Test :- Chemical Reactions And Equations Class –10 - Question 4
Development of bad smell and taste in food item containing oil / fat is due to :
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 4
Test :- Chemical Reactions And Equations Class –10 - Question 5
In the following chemical equation
Na2CO3 + xHCl → 2NaCl + CO2 + H2O.
The value of x is :
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 5
Test :- Chemical Reactions And Equations Class –10 - Question 6
Which one of the following process involves chemical reaction
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 6
Test :- Chemical Reactions And Equations Class –10 - Question 7
In the reaction,Mg + CuSO4 → MgSO4 + Cu :
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 7
Test :- Chemical Reactions And Equations Class –10 - Question 8
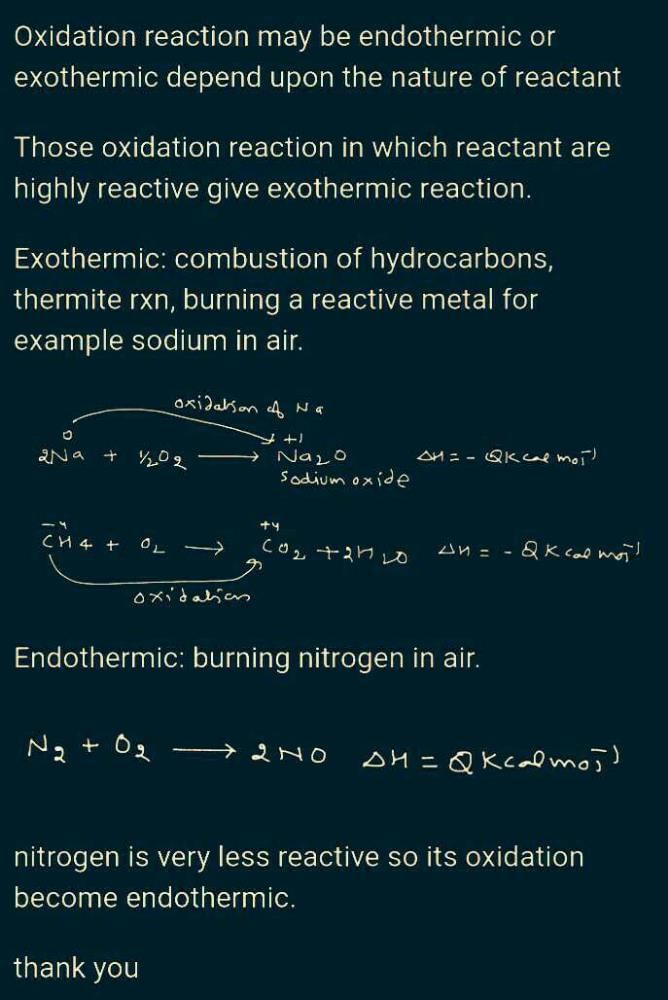
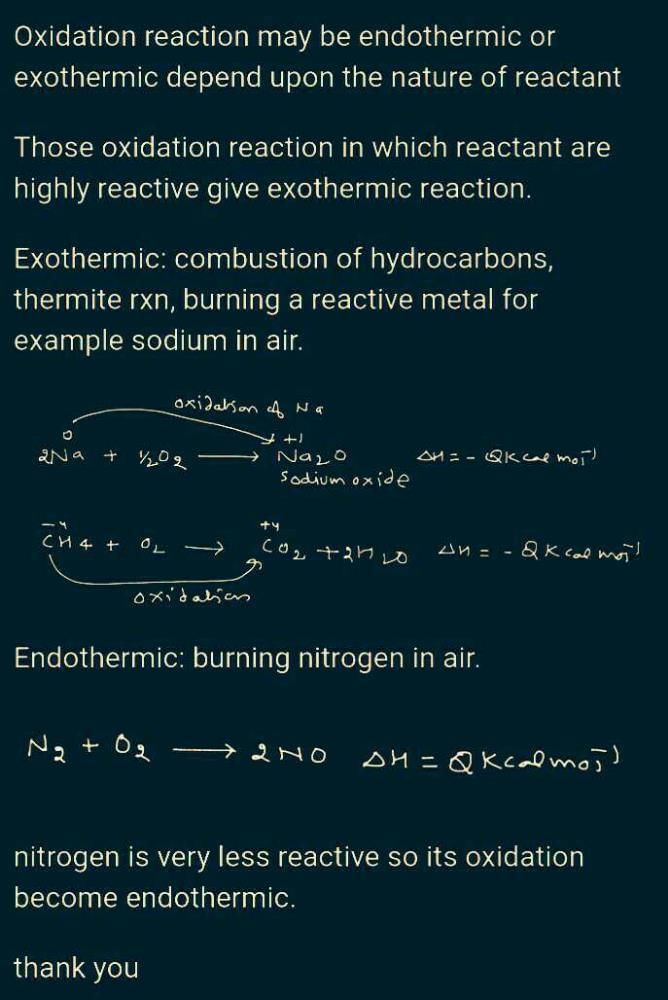
Which oxidising agent will you use to oxidise hydrogen sulphide ?
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 8
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 9
Test :- Chemical Reactions And Equations Class –10 - Question 10
Which metal fails to evolve hydrogen gas with dil. HCL ?
Detailed Solution for Test :- Chemical Reactions And Equations Class –10 - Question 10
Information about Test :- Chemical Reactions And Equations Class –10 Page
In this test you can find the Exam questions for Test :- Chemical Reactions And Equations Class –10 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test :- Chemical Reactions And Equations Class –10 , EduRev gives you an ample number of Online tests for practice
Download as PDF