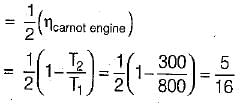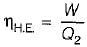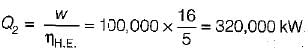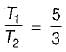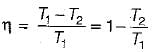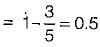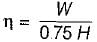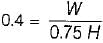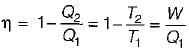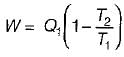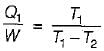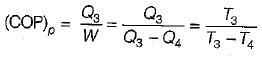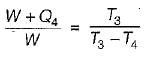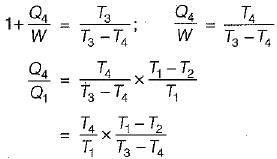Test: Second Law of Thermodynamics - 3 - Mechanical Engineering MCQ
10 Questions MCQ Test - Test: Second Law of Thermodynamics - 3
The Carnot cycle is unpredictable because it
An engine operates between temperature limits of 900 K and T2 and another between T2 and 400 K for both to be equally efficient T2 will be equal to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An inventor claims that a heat engine has the following specifications:
Developed = 50 kW
Fuel burned per hour = 3 kg
Heating value of fuel = 7500 kJ/kg
Temperature limit = 627°C and 27°C
Cost of fuel = Rs. 30/kg
Power the performance of his engine is
Developed = 50 kW
Fuel burned per hour = 3 kg
Heating value of fuel = 7500 kJ/kg
Temperature limit = 627°C and 27°C
Cost of fuel = Rs. 30/kg
Power the performance of his engine is
Three engines A, B and C operating on Carnot cycle respectively use air, steam and Helium as the working fluid. If all the engine operates within the same temperature limit, then which engine will have highest efficiency.
If a heat engine gives an output of 3 kW.when the input is 10000J/sec then the thermal efficiency of the engine will be
A Carnot engine operates between 27°C and 327°C. If the engine produces 300 kJ of work, what is the entropy change during heat rejection?
The following heat engine produces power of 100,000 kW. The heat engine operates between 800 K and 300 K. It has a thermal efficiency equal to 50% of that of the Carnot engine for the same temperatures. The rate at which heat is absorbed from the hot reservoir is
A heat engine operates at 75% of the maximum possible efficiency. The ratio of the heat source temperature (in K) to the heat sink temperature (in K) is 5/3. The fraction of the heat supplied that is converted to work is
Which of the following represents the Carnot cycle (ideal engine)?
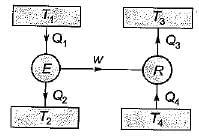
A reversible power cycle is used to drive a reversible heat pump cycle. The power cycle takes in Q1 heat units at T1 and reflects Q2 at T2. The heat pump abstracts Q4 from sink at T4 and discharges Q3 at T3. The ratio of Q4/Q1 (in terms of four temperature) is


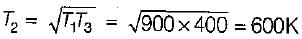

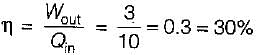
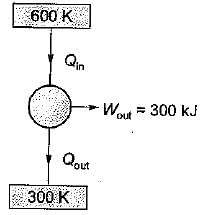
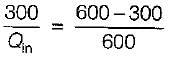
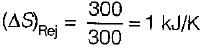
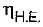 = 50% of Carnot cycle or engine
= 50% of Carnot cycle or engine