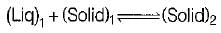Test: Equilibrium Diagrams & Phase Changes - 3 - Mechanical Engineering MCQ
30 Questions MCQ Test - Test: Equilibrium Diagrams & Phase Changes - 3
Martensite is the super saturated solution of carbon in
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a eutectic system, two elements are completely
During peritectic solidification, one liquid
Increase of ferrite phase in steel increases
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in
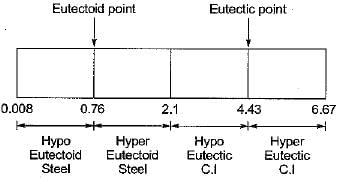
If a particular Fe-C alloy contains less than 0.83% carbon. It is called
Hyper eutectoid steels have structure of
Austenite is a solid solution of carbon in
Delta iron occur between the temperature range of
A material is said to be allotropic. If it has
In iron-carbide diagram, pearlite is
Match List-I (Name of material) with List-II (% Carbon Range) and select the correct answer using the codes given below the lists:
List-I
A. Hypoeutectoid steel
B. Hypereutectoid steel
C. Hypoeutectic cast iron
D. Hypereutectic cast iron
List-II
1. 4.3 — 6.67
2. 2.0 — 43
3. 0.8 — 2.0
4. 0.008 — 0.8
Codes:
A B C D
(a) 4 3 2 1
(b) 1 3 4 2
(c) 4 1 2 3
(d) 1 2 3 4
For good weldability, the carbon equivalent (%) of steel should be in the range of
Match List-I (Fe - Fe3 C Phase Diagram characteristic) with List-II (Phase) and select the correct answer using the codes given below the lists:
List-I
A. Alpha (α) iron
B. Iron carbide having crystal lattice
C. BCC pure allotropy of iron is stable between 1388°C and its melting point at 1535°C.
List-II
1. δ iron
2. Eutectic with 3 iron and 1 carbon atom
3. Ferrite stable between 1388°C
4. Cementite
Codes
A B C
(a) 4 2 3
(b) 3 4 1
(c) 4 2 1
(d) 3 1 2
As per Gibb's phase rule, if number of components is equal to 2 then the number of phases will be
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in
Eutectic composition of iron-carbon alloy always corresponds to its
As the weight percentage of carbon increases in plain carbon steel, its
In peritectoid reaction on cooling, we get one solid phase from
Consider the following lead-tin phase diagram given below:
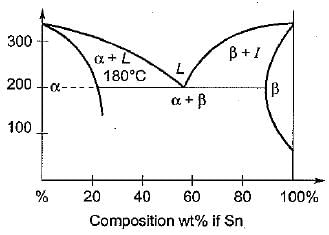
For which one of the following alloy compositions, the alloy wiil have the iowest melting point at 185°C?
Which one of the following sets of constituents is expected in equilibrium cooling of a hypereutectoid steel from austenitic state?
A given steel test specimen is studied under metallurgical microscope. Magnification used is 100 X. In that different phases are observed. One of them is Fe3C.
Which one of the following is the correct statements?
Which one of the following elements is a ferritic stabilizer?