Test: JEE Previous Year Questions- Solid State - JEE MCQ
7 Questions MCQ Test - Test: JEE Previous Year Questions- Solid State
The no. of atoms per unit cell in B.C.C. & F.C.C. is respectively -
[AIEEE-2002]
How many unit cells are present in a cube-shaped ideal crystal of NaCl of mass 1.00 g? [Atomic masses: Na = 23, Cl = 35.5]
[AIEEE-2003]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What type of crystal defect is indicated in the diagram below ?
[AIEEE-2004]
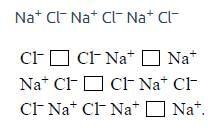
An ionic compound has a unit cell consisting of A ions at the corners of a cube and B ions on the centres of the faces of the cube. The empirical formula for this compound would be
[AIEEE-2005]
Copper crystallizes in fcc with a unit cell length of 361 pm. What is the radius of copper atom?
In a compound, atoms of element Y form ccp lattice and those of element X occupy 2/3rd of tetrahedral voids. The formula of the compound will be -
[AIEEE 2008]
Copper crystallises in fcc with a unit cell length of 361 pm. What is the radius of copper atom?


















