MCQ (Previous Year Questions) - Alcohols (Level 2) - MCQ
9 Questions MCQ Test - MCQ (Previous Year Questions) - Alcohols (Level 2)
1-propanol & 2-propanol can be best distinguished by - [JEE 2001]
Identify the correct order of boiling point of the following compounds : 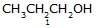
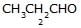
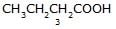 [JEE 2002]
[JEE 2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Product formed by P & Q can be differentiated by: [JEE 2003]
Reactn of entainomerically pure acid with 1 chiral carbon and racemic alcohol with 1 chiral carbon gives an ester which is - [JEE 2003]
+ Cl _ CH2CH2_CH3
P
Q + Phenol [JEE 2006]
The major products P and Q are -
Satement-1 : p-Hydroxybenzoic acid has a lower boiling point than o-hydroxybenzoic acid.
because
Statement-2 : o-Hydroxybenzoic acid has intramolecular hydrogen bonding. [JEE 2007]
(X)C5H13N (Y) (Tertiary alcohol + other products)
(Optically active)
Find X and Y. Is Y optically active ? Write the intermediate steps. [JEE 2005]
Column-I [IIT 2009]
(A) CH3CH2CH2CN (B) CH3CH2OCOCH3
(C) CH3_CH=CH_CH2OH (D) CH3CH2CH2CH2 NH2(s)
Column-II
(P) Reduction with Pd-C/H2
(Q) Reduction with SnCl2/HCl
(R) Development of foul smell o n treatment with chloroform and alcoholic KOH
(S) Reduction with diisobutylauminium hydride (DiBAL-H)
(T) Alkaline hydrolysis

















