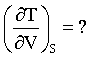Thermodynamics TEST - IIT JAM MCQ
30 Questions MCQ Test - Thermodynamics TEST
An ideal gas is expanding such that PT2 = constant. The coefficient of the volume expansion of the gas is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider the following reaction.
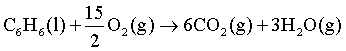
Signs of ΔH, ΔS and ΔG for the above reaction will be
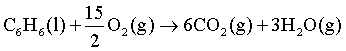
10 mole of ideal gas expand isothermally and reversibly from a pressure of 10 atm to 1 atm at 300 K. What is the largest mass which can lifted through a height of 100 meter?
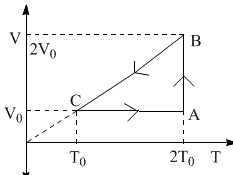
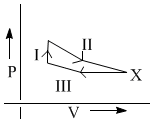
The efficiency of an ideal gas with adiabatic exponent ‘γ’ for the shown cyclic process would be:
 .
.
An ideal gas is at pressure P and temperature T in a box, which is kept in vacuum with in a large container. The wall of the box is punctured. What happens as the gas occupies entire container?
Initially one mole of ideal gas 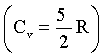 at 1.0 atm and 300 K is put through the following cycle:
at 1.0 atm and 300 K is put through the following cycle:
 .
.
Step – I : Heating to twice its initial pressure at constant volume.
Step – II : Adiabatic expansion to its initial temperature.
Step – III : Isothermal compression back to 1.00 atm.
What is the volume at state X?
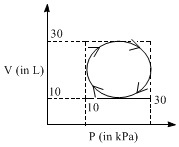
Heat absorbed by a system in going through a cyclic process shown in figure is:
 .
.
A heat engine carries one mole of an ideal mono-atomic gas around the cycle as shown in the figure. Select the correct option:
 .
.
An oil bath kept at 270C is being supplied heat at the rate of 100 js-1. Assuming the process to be quasi-static, the rate of increase of entropy of the system is approximately.
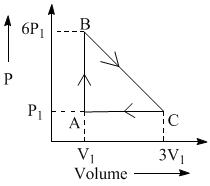
An ideal gas is taken around the cycle ABCA as shown in P – V diagram. The net work done during the cycle is equal to:
For the following reaction.
CO2 + H2O →H2CO3
The entropy change was calculate to be -96 JK-1 mol-1. The enthalpy change
was calculate to be -96 JK-1 mol-1. The enthalpy change  was measured to be -45kJ K-1 mol-1. This reaction is expected to be a spontaneous process. The total change in entropy
was measured to be -45kJ K-1 mol-1. This reaction is expected to be a spontaneous process. The total change in entropy 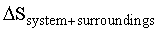 is:
is:
A heat engine absorbs 760 kJ of heat from a source at 380 K. it rejects
(i) 650 kJ (ii) 560 kJ
(iii) 504 kJ of heat to the sink at 280K state which of these represents respectively?
Which of the following properties are characteristics of an ideal solution?
One mole of a substance is heated from 300 K to 400 K at constant pressure. The of the substance is given by 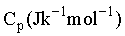 .The change in entropy, in
.The change in entropy, in 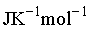 of the substance is ________________
of the substance is ________________
The Joule-Thomson Coefficient of carbon monoxide is at 298 K and 40.53 M Pa pressure, given that 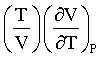 is 0.984, The Molar volume is 76 cm3 mol-1 and Cpm = 37.28 Jk-1 mol-1 :(in K atm-1)
is 0.984, The Molar volume is 76 cm3 mol-1 and Cpm = 37.28 Jk-1 mol-1 :(in K atm-1)
How many show intensive property:
Dipole-moment, refractive index, viscosity, surface tension, gas constant, chemical potential, vapour pressure, critical density
The molar residual entropy of a crystal in which the molecules can adopt 6 orientations of equal energy at 0K is(in jk-1mol-1)
A sample of an ideal gas is expanded 1 m3 to 3 m3 in a reversible process for which P = KV2 with K = 6 bar / m6. Work done by the gas is:(in KJ)
For C6H6(l), α = 1.24 × 10-3 K-1, and β = 9.228 × 10-4 MPa-1 at 298 K and 101.325 kPa pressure. Find the change in molar volume which will be required to produce an entropy change of 2.092 J K-1 mol-1 at 293 K. Assume α and β to be constants.(in cm3mol-1)
One mole of a van der Waals gas undergoes a change from 298 K and 1dm3 to 373 K and 10 dm3. What is the change in its entropy? Given b = 0.06 dm3 mol-1 , CV,m = 29.0 J K-1 mol-1(in JK-1mol-1)
Calculate the entropy change at 373 K for the transformatiom
H2O(l),( 1.01325 bar) = H2O(g)( 0.101325 bar)
Given: ΔvapH = 40.668 kJ mol-1(Answer in JK-1mol-1)
The molar heat capacities of Iodine vapour and solid are 7.8 and 14 cal/mol respectively if enthalpy of sublimation of iodine is 6096 cal/mole at 2000C, then what is ?U (internal energy change) at 2500C in cal/mol
20.0 dm3 of an ideal gas (diatomic, Cvm = 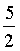 R) at 673K and 0.7 MPa expands until pressure of the gas is 0.2 M Pa. The Value of ΔH for the process if the expansion is carried out by adiabatic & reversible process.(in KJ)
R) at 673K and 0.7 MPa expands until pressure of the gas is 0.2 M Pa. The Value of ΔH for the process if the expansion is carried out by adiabatic & reversible process.(in KJ)


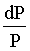 is equal to:
is equal to: