Test: Matter In Our Surroundings (Easy) - Class 9 MCQ
15 Questions MCQ Test - Test: Matter In Our Surroundings (Easy)
While mixing sugar with water, the level of water does not increase because
What is the effect on water's chemical and physical properties when the temperature is changed?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
During summer, water kept in an earthen pot becomes cool because of the phenomenon of
Meena experimented, as shown above. When she pushed the bottle into the basin of water, she could see bubbles escaping from the rubber tube into the beaker of water. What Can We infer from her experiment?
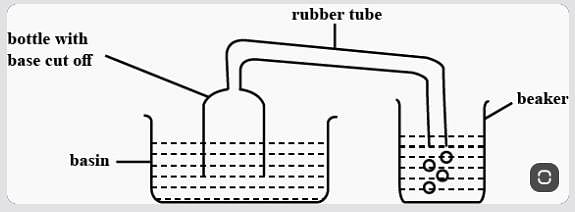
Which of the following is a surface phenomenon?
During change of state in a matter, the temperature remains the same due to:
The rate of evaporation does not depend on:
With the increase of temperature, which of these changes
Water Vapour At 373 K(100°C) have__________________________ energy than water at the same temperature.
The best evidence for the existence and movement of particles in liquids was provided by:
On placing an iron nail in a copper sulphate solution, it is observed that :
Rahul added 2 mL of barium chloride solution to 2 mL sodium sulphate solution in a test tube and observed that :
While determining the boiling point of water, the teacher suggested adding some pumice stone pieces to the hard glass test tube containing water. This was done to :
To determine the melting point of ice, a student immersed the thermometer bulb in crushed ice in a beaker and heated the beaker on a low flame. He would observe :

















