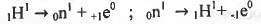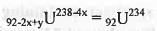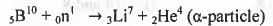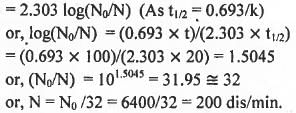Test: Radioactivity - IIT JAM MCQ
8 Questions MCQ Test - Test: Radioactivity
The decay modes of 14C and ,14O are
1. β-decay ;
2. positron emission ;
3. β-decay and positron emission, respectively ;
4. positron emission and β-decay, respectively
[2018]
The no. of α and β particle(s), generated in the following radioactive decay process are :
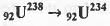
[2014]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In boron neutron capture therapy, the initial boron isotope used and the particle generated after neutron capture respectively are :
1. 11B and α particle ;
2. 10B and α particle ;
3. 11B and β particle ;
4. 11B and β particle.
[2014]
The total no. of steps involved and no. of beta particles emitted in the spontaneous decay of 99U238 → 82Pb206 respectively, are
[2012]
In the following equation X is
95Am241 + α → 97Bk243 + X
[2011]
The half-life of a radioactive nuclide is 20 years. If a sample of this nuclide has an activity of 6400 dis/min today, the activity after 100 years would be
[2006]
The radioactive isotope used to locate brain tumours is
[2005]