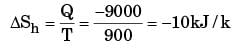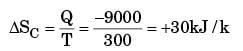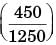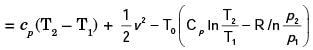Past Year Questions: Availability And Irreversibility - Mechanical Engineering MCQ
6 Questions MCQ Test - Past Year Questions: Availability And Irreversibility
A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is
[1995]
Availability of a system at any given state is
[2000]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Considering the relationship TdS = dU + pdV between the entropy (S), internal energy (U), pressure (p), temperature (T) and volume (V), which of the following statements is correct?
[2003]
A steel billet of 2000 kg mass is to be cooled from 1250 K to 450 K. The heat released during this process is to be used as a source of energy. The ambient temperature is 303 K and specific heat of steel is 0.5 kJ/kgK. The available energy of this billet is
[2004]
The pressure, temperature and velocity of air flowing in pipe are 5 bar, 500 K and 50 m/s, respectively. The specific heats of air at a constant pressure and at constant volume are 1.005 kJ/kgK and 0.718 kJ/kgK, respectively.Neglect potential energy. If the pressure and temperature of the surroundings are 1 bar and 300 K, respectively, the available energy in kJ/ kg of the air stream is
[2013]
The maximum theoretical work obtainable, when a system interacts to equilibrium with a reference environment, is called
[2014]