Case Based Questions Test: Acids, Bases & Salts - 2 - Class 10 MCQ
15 Questions MCQ Test - Case Based Questions Test: Acids, Bases & Salts - 2
Read the following and answer any questions:
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released.
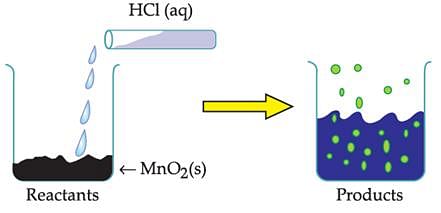
Chlorine gas reacts with _______ to form bleaching powder.

Read the passage and answer the following questions.
Suhana wanted her house to be white washed. She bought 10 kg of quicklime from the market and dissolved it in 30 L of water. On adding lime to water, she observed that the water started boiling even when it was not being heated.
Name the product when water is added to quicklime.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct formula for calcium hydroxide is:
The common name for quick lime is:
Read the passage and answer the following questions:
Sanjana while preparing the cake used baking soda in small amounts. It helps to make the cake soft and spongy. An aqueous solution of baking soda also turns red litmus blue. It is also used in soda acid extinguishers.
Name the gas produced by the reaction of baking soda and acid which helps as fire extinguisher:
The pH of baking soda solution is:
What is the chemical name for baking soda?
Read the passage and answer the following questions.
A dry pellet of a common base B when kept in open absorbs moisture and turns sticky. The compound is also a by-product of the chlor-alkali process.
Identify B:
What is the raw material used in chloro-alkali?
pH is quite useful to us in a number of ways in daily life. Some of its applications are:
Control of pH of the soil: Plants need a specific pH range for proper growth. The soil may be acidic, basic or neutral depending upon the relative concentration of H* and OH-. The pH of any soil can be determined by using pH paper. If the soil is too acidic, it can be corrected by adding lime to it. If the soil is too basic, it can be corrected by adding organic manure which contains acidic materials.
Regaining shine of a tarnished copper vessel by use of acids: A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lenion on the vessel, the surface is cleaned and the vessel begins to shine again. This is due to the fact that copper oxide is basic in nature, which reacts with the acid (citric acid) present in lemon to form a salt (copper citrate) which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed.
Self-defence by animals through chemical warfare: Stings of bees and ants contain methanoic acid. When stung, it causes lot of pain and irritation. This can be cured by rubbing the affected area with mild base like baking soda.
Q. P is an aqueous solution of acid and Q is an aqueous solution of base. When these two are diluted separately, then
When aqueous sodium carbonate (Na2CO3) reacts with HCl (aq), it gives
The primary reason behind the formation of the toxic foam is high phosphate content in the wastewater because of detergents used in dyeing industries, dhobi ghat Yamuna’s pollution level is so bad that parts of it have been labelled ‘dead’ as there is no oxygen in it for aquatic life to survive.

Which of the following statements is correct for the water with detergents dissolved in it?
High content of phosphate ion in river Yamuna may lead to:
Study the given table and answer the following questions. It shows the pH value of the plaque (which collects around teeth) surrounding the teeth of a child over 3 hrs.

The pH which leads to tooth decay?
Suhana takes three beakers A, B and C filled with aqueous solutions of glucose, alcohol and hydrochloric acid respectively as shown in the following figure:
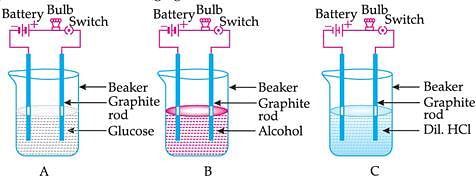
The bulb glows in a solution depending on whether the solution is:

















