Case Based Questions Test: Metals & Non-metals - 1 - Class 10 MCQ
15 Questions MCQ Test - Case Based Questions Test: Metals & Non-metals - 1
Study the given table and answer the questions:
A student took the samples of four metals A, B, C and D and added the following solutions one by one. The results obtained have been tabulated as follows:
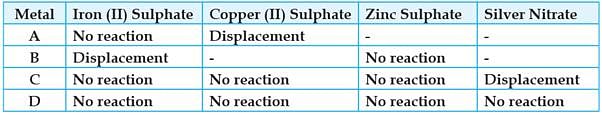
Choose the most reactive metal:

Study the given table and answer the questions:
A student took the samples of four metals A, B, C and D and added the following solutions one by one. The results obtained have been tabulated as follows:
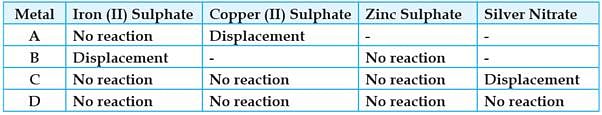
Which of the following will displace Cu from its solution of sulphate:

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Study the given table and answer the questions:
A student took the samples of four metals A, B, C and D and added the following solutions one by one. The results obtained have been tabulated as follows:
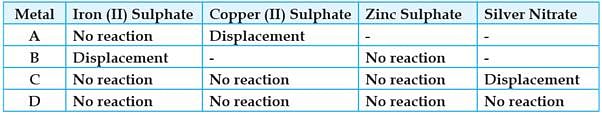
On the basis of sequence of reactions, identify the most and least reactive elements.
A + BX → AX + B
C + AY → CY + A

When a silvery grey powder of a solid (A) is mixed with a powder of solid (B) no reaction occurs. But if the mixture is ignited and lighted using magnesium ribbon a reaction occurs with the evolution of a large amount of heat forming product (C) which settles down as liquid metal and the solid product (D) formed floats on the liquid (C). (C) in solid form reacts with moisture to form rust. The amount of heat generated during the reaction is so high that the reaction is used in welding of electric conductors, joints in railway tracks. Based on this information, answer the questions.
Identify A and C?
When a silvery grey powder of a solid (A) is mixed with a powder of solid (B) no reaction occurs. But if the mixture is ignited and lighted using magnesium ribbon a reaction occurs with the evolution of a large amount of heat forming product (C) which settles down as liquid metal and the solid product (D) formed floats on the liquid (C). (C) in solid form reacts with moisture to form rust. The amount of heat generated during the reaction is so high that the reaction is used in welding of electric conductors, joints in railway tracks. Based on this information, answer the questions.
Identify B and D which are oxides of:
When a silvery grey powder of a solid (A) is mixed with a powder of solid (B) no reaction occurs. But if the mixture is ignited and lighted using magnesium ribbon a reaction occurs with the evolution of a large amount of heat forming product (C) which settles down as liquid metal and the solid product (D) formed floats on the liquid (C). (C) in solid form reacts with moisture to form rust. The amount of heat generated during the reaction is so high that the reaction is used in welding of electric conductors, joints in railway tracks. Based on this information, answer the questions.
The reaction in which heat is generated is called as:
Read the following passage and answer the questions.
Sohan went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was sad but after a futile argument, the man beat a hasty retreat.
Which of the following is used for dissolution of gold?
Read the following passage and answer the questions.
Sohan went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was sad but after a futile argument, the man beat a hasty retreat.
The composition of aqua-regia is
Read the following passage and answer the questions.
Sohan went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was sad but after a futile argument, the man beat a hasty retreat.
Examples of Noble metals are:
Read the following passage and answer the questions.
During extraction of metals, electrolytic refining is used to obtain pure metals. During the process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. The solution of the metal salt is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the anode dissolves from the electrolyte. An equivalent of pure metal from the electrolyte is deposited on the cathode.
The process of purification of the metal obtained after reduction, is called:
Read the following passage and answer the questions.
During extraction of metals, electrolytic refining is used to obtain pure metals. During the process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. The solution of the metal salt is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the anode dissolves from the electrolyte. An equivalent of pure metal from the electrolyte is deposited on the cathode.
Which of the metals are refined by electrolytic refining?
I. Au
II. Cu
III. Na
IV. K
Read the following passage and answer the questions.
During extraction of metals, electrolytic refining is used to obtain pure metals. During the process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. The solution of the metal salt is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the anode dissolves from the electrolyte. An equivalent of pure metal from the electrolyte is deposited on the cathode.
Anode is _______electrode while cathode is ---------electrode:
Choose the correct option.
Read the following passage and answer the questions.
When a silvery grey powder of a solid (A) is mixed with a powder of solid (B) no reaction occurs. But if the mixture is ignited and lighted using magnesium ribbon a reaction occurs with evolution of a large amount of heat forming product (C) which settles down as liquid metal and the solid product (D) formed floats on the liquid (C). (C) in solid form reacts with moisture to form rust. The amount of heat generated during the reaction is so high that the reaction is used in welding of electric conductors, joints in railway tracks. Based on this information, answer the questions.
Which of the following is amphoteric in nature:
Read the following passage and answer the questions.
When a silvery grey powder of a solid (A) is mixed with a powder of solid (B) no reaction occurs. But if the mixture is ignited and lighted using magnesium ribbon a reaction occurs with evolution of a large amount of heat forming product (C) which settles down as liquid metal and the solid product (D) formed floats on the liquid (C). (C) in solid form reacts with moisture to form rust. The amount of heat generated during the reaction is so high that the reaction is used in welding of electric conductors, joints in railway tracks. Based on this information, answer the questions.
Amphoteric oxides are:
Read the following passage and answer the questions.
During extraction of metals, electrolytic refining is used to obtain pure metals. During the process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. The solution of the metal salt is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the anode dissolves from the electrolyte. An equivalent of pure metal from the electrolyte is deposited on the cathode.
During electrolytic refining of zinc, it gets