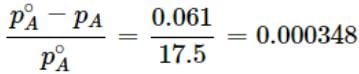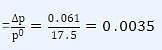Case Based Questions Test: Solutions - NEET MCQ
15 Questions MCQ Test - Case Based Questions Test: Solutions
Directions : Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q. Relative lowering of vapour pressure for the given solution is
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q. Relative lowering of vapour pressure for the given solution is
Directions : Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q. Mole fraction of sugar in the solution is
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q. Mole fraction of sugar in the solution is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions : Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q.The vapour pressure (mm of Hg) of solution will be
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q.The vapour pressure (mm of Hg) of solution will be
Directions : Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 200C is 17.5 mm of Hg).
Q. The vapour pressure (mm of Hg) of water at 293K when 25g of glucose is dissolved in 450 g of water is
Directions : Read the passage given below and answer the following questions :
Scuba apparatus includes a tank of compressed air toted by the diver on his or her back, a hose for carrying air to a mouthpiece, a face mask that covers the eyes and nose, regulators that control air flow, and gauges that indicate depth and how much air remains in the tank.
A diver who stays down too long, swims too deep, or comes up too fast can end up with a condition called “the bends.” In this case, bubbles of gas in the blood can cause intense pain, even death.
In these following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Scuba divers may face a medical condition called ‘bends’.
Reason : ‘Bends’ can be explained with the help of Henry’s law as it links the partial pressure of gas to that of its mole fraction.
Directions : Read the passage given below and answer the following questions :
Scuba apparatus includes a tank of compressed air toted by the diver on his or her back, a hose for carrying air to a mouthpiece, a face mask that covers the eyes and nose, regulators that control air flow, and gauges that indicate depth and how much air remains in the tank.
A diver who stays down too long, swims too deep, or comes up too fast can end up with a condition called “the bends.” In this case, bubbles of gas in the blood can cause intense pain, even death.
In these following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Soft drinks and soda water bottles are sealed under high pressure.
Reason : High pressure maintains the taste and texture of the soft drinks.
Directions : Read the passage given below and answer the following questions :
Scuba apparatus includes a tank of compressed air toted by the diver on his or her back, a hose for carrying air to a mouthpiece, a face mask that covers the eyes and nose, regulators that control air flow, and gauges that indicate depth and how much air remains in the tank.
A diver who stays down too long, swims too deep, or comes up too fast can end up with a condition called “the bends.” In this case, bubbles of gas in the blood can cause intense pain, even death.
In these following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Anoxia is a condition experienced by climbers which makes them suddenly agile and unable to think clearly.
Reason : At high altitudes the partial pressure of oxygen is less than that at the ground level.
Directions : Read the passage given below and answer the following questions :
Scuba apparatus includes a tank of compressed air toted by the diver on his or her back, a hose for carrying air to a mouthpiece, a face mask that covers the eyes and nose, regulators that control air flow, and gauges that indicate depth and how much air remains in the tank.
A diver who stays down too long, swims too deep, or comes up too fast can end up with a condition called “the bends.” In this case, bubbles of gas in the blood can cause intense pain, even death.
In these following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Bends is caused due to formation of nitrogen bubbles in the blood of scuba divers which blocks the capillaries.
Reason : Underwater high pressure increases solubility of gases in blood, while as pressure gradually decreases moving towards the surface, gases are released and nitrogen bubbles are formed in blood.
Directions : Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2

Q. What type of deviation from Raoult’s law does the above graph represent ?
Directions : Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2

Q. A solution of two liquids boils at a temperature more than the boiling point of either of them. What type of deviation will be shown by the solution formed in terms of Raoult’s law ?
Directions : Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2
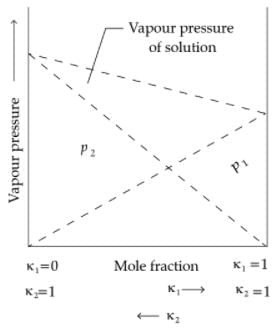
Q. Which of the following aqueous solutions should have the highest boiling point ?
Directions : Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2
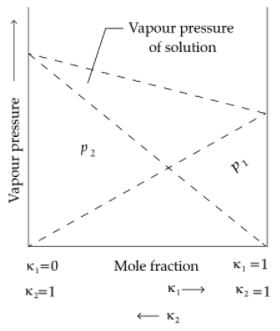
Q. In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.
Direction: Read the passage given below and answer the following questions:
Boiling point or freezing point of liquid solution would be affected by the dissolved solids in the liquid phase. A soluble solid in solution has the effect of raising its boiling point and depressing its freezing point. The addition of non-volatile substances to a solvent decreases the vapour pressure and the added solute particles affect the formation of pure solvent crystals.
According to many researches the decrease in freezing point directly correlated to the concentration of solutes dissolved in the solvent. This phenomenon is expressed as freezing point depression and it is useful for several applications such as freeze concentration of liquid food and to find the molar mass of an unknown solute in the solution. Freeze concentration is a high quality liquid food concentration method where water is removed by forming ice crystals. This is done by cooling the liquid food below the freezing point of the solution. The freezing point depression is referred as a colligative property and it is proportional to the molar concentration of the solution (m), along with vapour pressure lowering, boiling point elevation, and osmotic pressure. These are physical characteristics of solutions that depend only on the identity of the solvent and the concentration of the solute. The characters are not depending on the solute’s identity.
Q. When a non volatile solid is added to pure water it will:
Direction: Read the passage given below and answer the following questions:
Boiling point or freezing point of liquid solution would be affected by the dissolved solids in the liquid phase. A soluble solid in solution has the effect of raising its boiling point and depressing its freezing point. The addition of non-volatile substances to a solvent decreases the vapour pressure and the added solute particles affect the formation of pure solvent crystals.
According to many researches the decrease in freezing point directly correlated to the concentration of solutes dissolved in the solvent. This phenomenon is expressed as freezing point depression and it is useful for several applications such as freeze concentration of liquid food and to find the molar mass of an unknown solute in the solution. Freeze concentration is a high quality liquid food concentration method where water is removed by forming ice crystals. This is done by cooling the liquid food below the freezing point of the solution. The freezing point depression is referred as a colligative property and it is proportional to the molar concentration of the solution (m), along with vapour pressure lowering, boiling point elevation, and osmotic pressure. These are physical characteristics of solutions that depend only on the identity of the solvent and the concentration of the solute. The characters are not depending on the solute’s identity.
Q. Assume three samples of juices A, B and C have glucose as the only sugar present in them.
The concentration of sample A, B and C are 0.1M, .5M and 0.2 M respectively. Freezing point will be highest for the fruit juice:
Direction: Read the passage given below and answer the following questions:
Boiling point or freezing point of liquid solution would be affected by the dissolved solids in the liquid phase. A soluble solid in solution has the effect of raising its boiling point and depressing its freezing point. The addition of non-volatile substances to a solvent decreases the vapour pressure and the added solute particles affect the formation of pure solvent crystals.
According to many researches the decrease in freezing point directly correlated to the concentration of solutes dissolved in the solvent. This phenomenon is expressed as freezing point depression and it is useful for several applications such as freeze concentration of liquid food and to find the molar mass of an unknown solute in the solution. Freeze concentration is a high quality liquid food concentration method where water is removed by forming ice crystals. This is done by cooling the liquid food below the freezing point of the solution. The freezing point depression is referred as a colligative property and it is proportional to the molar concentration of the solution (m), along with vapour pressure lowering, boiling point elevation, and osmotic pressure. These are physical characteristics of solutions that depend only on the identity of the solvent and the concentration of the solute. The characters are not depending on the solute’s identity.
Q. Colligative properties are: