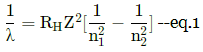Assertion & Reason Test: Atoms - Grade 12 MCQ
11 Questions MCQ Test - Assertion & Reason Test: Atoms
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): According to Rutherford, the atomic model, the path of electrons is parabolic.
Reason (R): Rutherford could not explain the stability of atoms.
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Bohr model is not applicable for multi-electron models.
Reason (R): Bohr model cannot account for sublevel (s, p, d, f) orbitals and electron spin.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Bohr postulated that the electrons in stationary orbits around the nucleus do not radiate.
Reason (R): According to classical Physics, all moving electrons radiate.
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): In the α-particle scattering experiment, most of the α-particles pass undeviated.
Reason (R): Most of the space in the atom is empty.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : According to classical theory the proposed path of an electron in Rutherford atom model will be parabolic.
Reason : According to electromagnetic theory an accelerated particle continuously emits radiation.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Electrons in the atom are held due to Coulomb forces.
Reason : The atom is stable only because the centripetal force due to Coulomb’s law is balanced by the centrifugal force.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Between any two given energy levels, the number of absorption transitions is always less than the number of emission transitions.
Reason : Absorption transitions start from the lowest energy level only and may end at any higher energy level. But emission transitions may start from any higher energy level and end at any energy level below it.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : The force of repulsion between atomic nucleus and α-particle varies with distance according to inverse square law.
Reason : Rutherford did α-particle scattering experiment.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Bohr had to postulate that the electrons in stationary orbits around the nucleus do not radiate.
Reason: According to classical physics all moving electrons radiate.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Hydrogen atom consists of only one electron but its emission spectrum has many lines.
Reason : Only the Lyman series is found in the absorption spectrum of hydrogen atoms whereas in the emission spectrum, all the series are found.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4
Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.