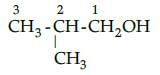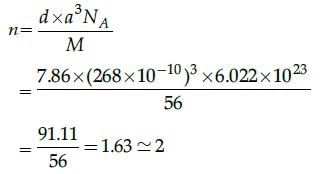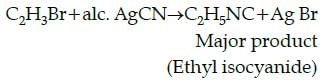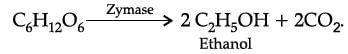Test: Class 12 Chemistry: CBSE Sample Question Paper- Term I (2021-22) - Class 12 MCQ
30 Questions MCQ Test - Test: Class 12 Chemistry: CBSE Sample Question Paper- Term I (2021-22)
The IUPAC name of isobutyl alcohol is :
Formation of ortho hydroxy benzoic acid from phenol using sodium hydroxide is:
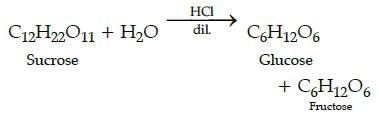
Glucose and fructose are prepared in equal amount when:
Which is true for crystalline solids:
All the elements of group 15 form EX3 and EX5 except:
When constituent particles are present only on the corner positions of a unit cell:
Chloroform mixed with nitrogen gas is a type of which solution:
What is incorrect about fibrous proteins:
Name the following structures of proteins:


Which of the following is not a haloalkane ?
In solid alkali halides, the appearance of colour is due to:
Supersonic jet aeroplanes are responsible for depleting the conc. of ozone layer because:
Which of the following does not react with oxygen directly ?
In ______the domains are oppositely oriented and cancel out each other’s magnetic moment :
Which of the following is not a chiral compound :
For any solution the partial vapour pressure of each volatile component in the solution is directlyproportional to its_______________.
The value of van’t Hoff factors for K2SO4, NaCl and KCl is :
Many wet gases are dried by passing through sulphuric acid, this shows sulphuric acid is:
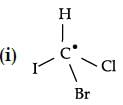
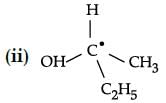
Choose the molecules in which carbon atom marked with asterisk (*) is asymmetric ?

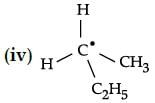
Calculate the number of atoms per unit cell of iron crystal lattice if the edge length is 268 pm anddensity is 7. 86 g / cm3: [Molar mass of Fe = 56 g / mol].
How much amount of NaCl in gram will be required for the preparation of its 5,6 w/v 1000 mlaqueous solution ?
Fuming nitric acid is a mixture of:
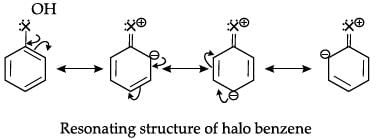
The presence of halogen atom in the haloarenes directs the incoming substituent in electrophilessubstitution reactions to:
Give the major product in the following reaction:
C2H5Br + alc. AgCN → Product
Glucose is converted into ethanol in the persence of:
Which allotropic form of sulphur is most stable at room temperature:
The number of sulphur molecules in monoclinic and rhombic sulphur:
Sulphur dioxide when passed through water forms:
Choose the correct answer from the options given:
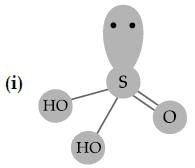
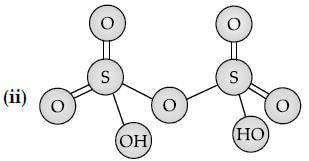
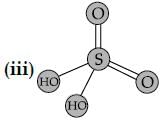
Sulphuric acid is used to manufacture more volatile acids from their corresponding salts due to its: