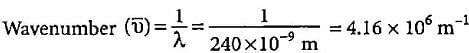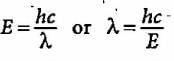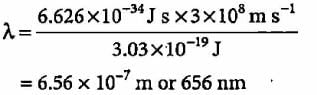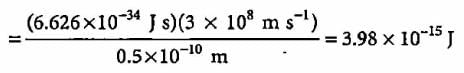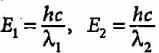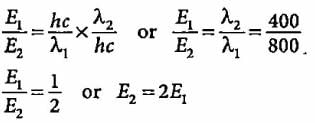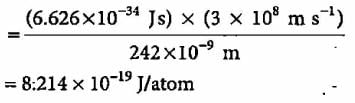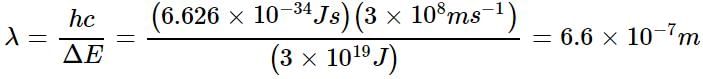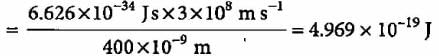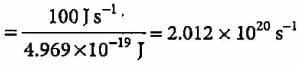Test: Developments leading to Bohr's Model of Atom - NEET MCQ
15 Questions MCQ Test - Test: Developments leading to Bohr's Model of Atom
What will be the wavenumber of yellow radiation having wavelength 240 nm?
What will be the energy of one photon of radiation whose frequency is 5 × 1014 Hz?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The energy of a photon is given as 3.03 x 10-19 J/atom. The wavelength of the photon is
What will be the energy of a photon which corresponds to the wavelength of 0.50 Å?
Compare the energies of two radiations E1 with wavelength 800 nm and E2 with wavelength 400 nm.
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionise the sodium atom. What is the ionisation energy of sodium per atom?
The energy difference between the ground state of an atom and its excited state is 3 x 10-19 J. What is the wavelength of the photon required for this transition?
A 100 watt bulb emits monochromatic light of wavelength 400 nm. Calculate the number of photons emitted per second by the bulb.
Mark the incorrect statement regarding the photoelectric effect.
A certain metal when irradiated by light (v = 3.2 x 1016 Hz) emits photoelectrons with twice K.E. as did photoelectrons when the same metal is irradiated by light (v = 2.0 x 1016 Hz). The v0 of the metal is
The spectrum of white light ranging from red to violet is called a continuous spectrum because
The emission spectrum of hydrogen is found to satisfy the expression for the energy change ΔE (in joules) such that 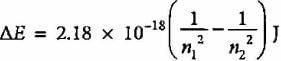 where n1 = 1, 2, 3,.... and n2 = 2, 3, 4. The spectral lines corresponds to Paschen series if
where n1 = 1, 2, 3,.... and n2 = 2, 3, 4. The spectral lines corresponds to Paschen series if
The wavelength of visible light is
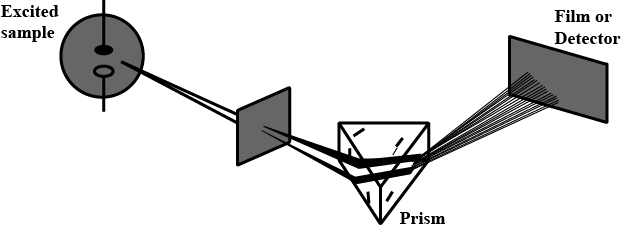
Which of the following types of spectrum is best depicted by the given figure?
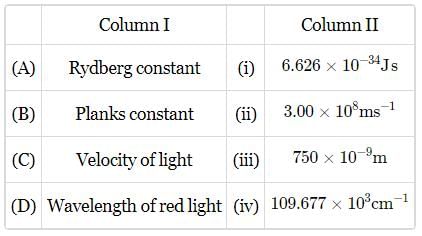
Match the constants given in column I with their values given in column II and mark the appropriate, choice.