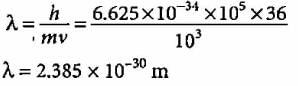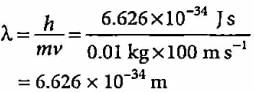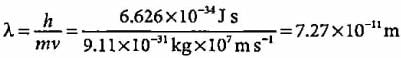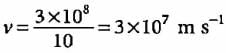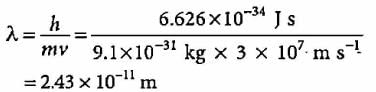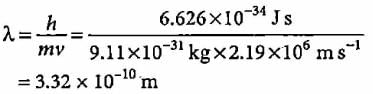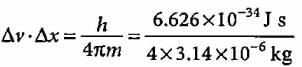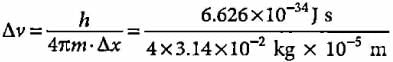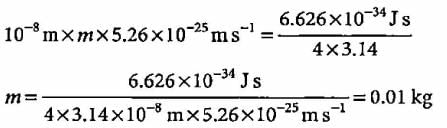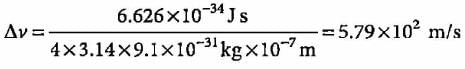Test: Quantum Mechanical Model of Atom - 1 - NEET MCQ
10 Questions MCQ Test - Test: Quantum Mechanical Model of Atom - 1
The de Broglie wavelength associated with a ball of mass 200 g and moving at a speed of 5 metres/hour, is of the order of (h = 6.625 x 10-34 J s) is
A body of mass 10 g is moving with a velocity of 100 m s-1. The wavelength associated with it is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The wavelength of an electron moving with velocity of 107 m s-1 is
What will be the wavelength of an electron moving with 1/10th of velocity of light?
If the velocity of an electron in Bohr’s first orbit is 2.19 x 106 m s-1, what will be the de Broglie wavelength associated with it?
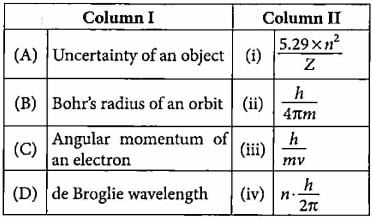
Match the column I with column II and mark the appropriate choice.
If uncertainty principle is applied to an object of mass 1 milligram, the uncertainty value of velocity and position will be
What will be the uncertainty in velocity of a bullet with a mass of 10 g whose position is known with ± 0.01 mm?
What will be the mass of a particle if uncertainty in its position is 10-8 m and velocity is 5.26 x 10-25 m s-1?
What will be the uncertainty in velocity of an electron when the uncertainty in its position is 1000 Å?