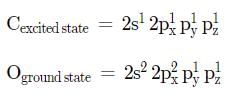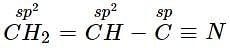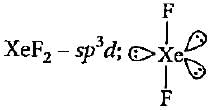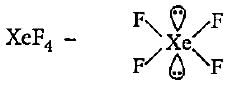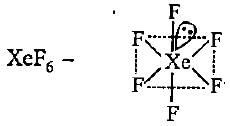Test: Hybridisation (NCERT) - NEET MCQ
15 Questions MCQ Test - Test: Hybridisation (NCERT)
Which of the following statements is true about hybridisation?
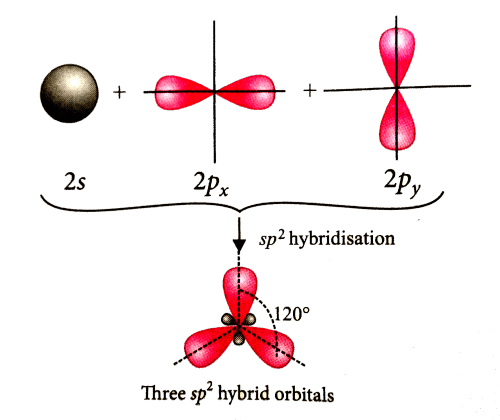
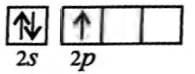
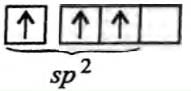
Which of the following molecules is formed by sp2 hybrid orbitals?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which type of hybridisation is shown by carbon atoms from left to right in the given compound;
CH2 = CH — C ≡ N?
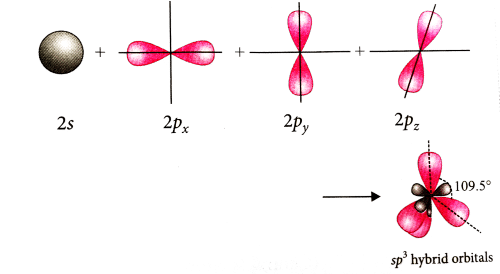
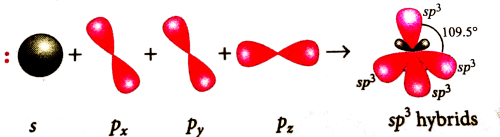
On hybridisation of one s and three p-orbitals, we get
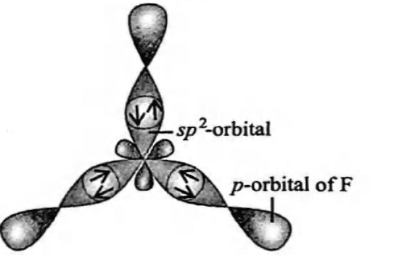
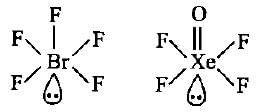
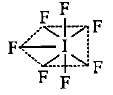
The molecules like BrF5 and XeOF4 are square pyramidal in shape.What is the type of hybridisation shown in these molecules?
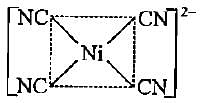
Which of the following shows dsp2 hybridisation and a square planar geometry?
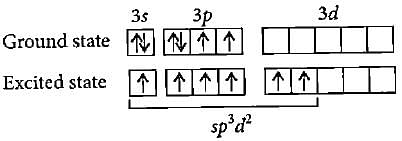
Hybridisation state of Xe in XeF2, XeF4 and XeF6 respectively are
What is the hybrid state of carbon in ethyne, graphite and diamond?
Given below is the bond angle in various types of hybridisation. Mark the bond angle which is not correctly matched.
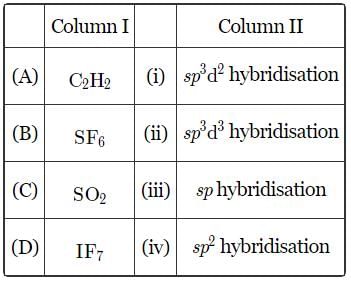
Match the column I with column II and mark the appropriate choice.
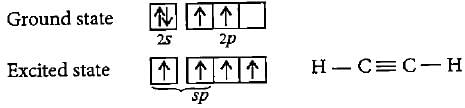
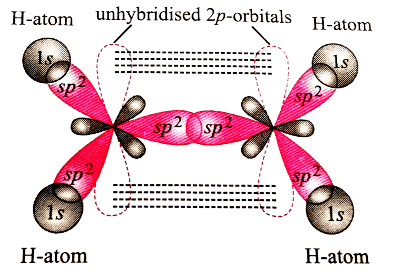
In formation of ethene, the bond formation between s and p-orbitals takes place in the following manner.
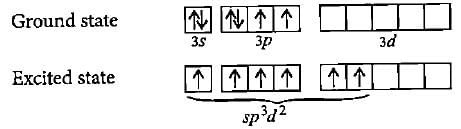
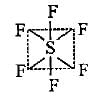
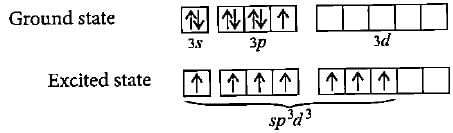
The ground state electronic configuration of S is 3s23p4. How does it form the compound SF6?