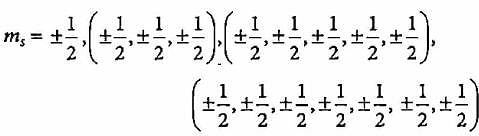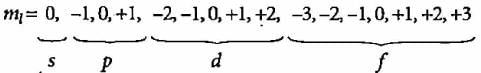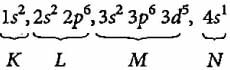Test: Quantum Mechanical Model of Atom - 2 - NEET MCQ
30 Questions MCQ Test - Test: Quantum Mechanical Model of Atom - 2
The probability of finding out an electron at a point within an atom is proportional to the
Two electrons present in M shell will differ in
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the lowest value of n that allows g orbital to exist?
How many subshells and electrons are associated with n = 4?
An electron is in one of the 3d-orbitals. What are the possible values of n, l and m for this electron?
What are the possible values of n, l and ml for an atomic orbital 4f?
Describe the orbital with following quantum numbers:
(i) n = 3, l = 2
(ii) n = 4 , l = 3
How many electrons in an atom have the following quantum numbers?
n = 4, s = -1/2
In how many elements the last electron will have the following set of quantum numbers, n = 3 and l = 1?
An orbital is described with the help of a wave function. Since many wave functions are possible for an electron, there are many atomic orbitals. When atom is placed in a magnetic field the possible number of orientations for an orbital of azimuthal quantum number 3 is:
What will be the orbital angular momentum of an electron in 2s-orbital?
Two values of spin quantum numbers i.e., +1/2 and -1/2 represent
The 3d-orbitals having electron density in all the three axes is
The number of radial nodes and angular nodes for d-orbital can be represented as
Few statements are given regarding nodes in the orbitals. Mark the statement which is not correct.
Which of the following is not a correct statement regarding the energies of orbitals?
Few electrons have following quantum numbers,
(i) n = 4, l = 1
(ii) n = 4, l = 0
(iii) n = 3, l = 2
(iv) n = 3, l = 1
Arrange them in the order of increasing energy from lowest to highest.
An electron can enter into the orbital when
How many orbitals in total are associated with 4th energy level?
The orbital diagram in which the Aufbau principle is violated is
Which of the sequences given below shows the correct increasing order of energy?
What is the electronic configuration of O2- ion?
Which atom (X) is indicated by the following configuration?
X → [Ne] 3s2 3p3
Which of the following configurations represents the most electronegative element?
Which of the following configurations represents a noble gas?
An element has 13 electrons in its M shell and 1 electron in N shell in ground state. Identify the element.
The configuration of the valence orbital of an element with atomic number 22 is
Which of the following quantum numbers are correct for the outermost electron of sodium atom?
Which of the following configurations does not follow Hund’s rule of maximum multiplicity?
Read the following statements and mark the incorrect statement.