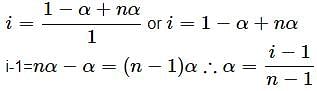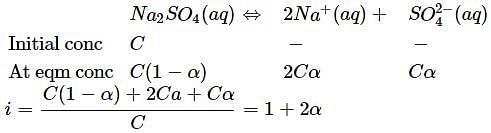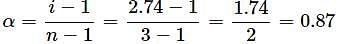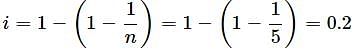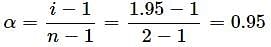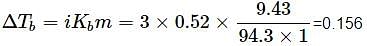Test: Abnormal Molar Masses (NCERT) - NEET MCQ
15 Questions MCQ Test - Test: Abnormal Molar Masses (NCERT)
Why is the molecular mass determined by measuring colligative property in case of some solutes is abnormal?
Which of the following representations of i (van't Hoff factor) is not correct?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following relations is not correctly matched with the formula?
Which of the following will have same value of vant's Hoff factor as that of K4[Fe(CN)6]?
Arrange the following aqueous solutions in the order of their increasing boiling points
(i) 10−4M NaCl
(ii) 10−4M Urea
(iii) 10−3M MgCl2
(iv) 10−2M NaCl
Which of the following has the highest freezing point?
If α is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is:
For which of the following solutes the van’t Hoff factor is not greater than one?
What will be the degree of dissociation of 0.1M Mg(NO3)2 solution if van't Hoff factor is 2.74?
Which of the following will have the highest f.pt. at one atmosphere?
A solute X when dissolved in a solvent associates to form a pentamer. The value of van't Hoff factor (i) for the solute will be
What will be the freezing point of a 0.5m KCl solution? The molal freezing point constant of water is 1.86∘C m−1.
What amount of CaCl2 (i = 2.47) is dissolved in 2 litres of water so that its osmotic pressure is 0.5atm at 27oC?
The van't Hoff factor of a 0.005 M aqueous solution of KCl is 1.95. The degree of ionisation of KCl is
The elevation in boiling point of a solution of 9.43g of MgCl2 in 1kg1kg of water is (Kb = 0.52 K kg mol−1, Molar mass of MgCl2 = 94.3 g mol−1)