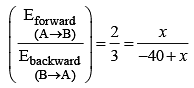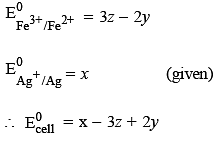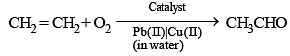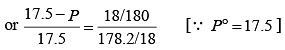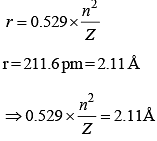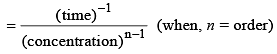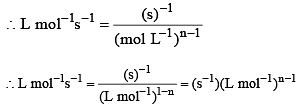NEET Practice Test - 12 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 12
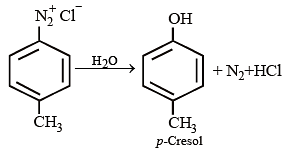
Which of the following reagents will convert p-methyl benzenediazonium chloride into p-cresol?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a hydrogen atom, if energy of an electron in ground state is – 13.6. eV, then energy in the 2nd excited state is
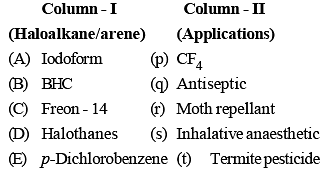
Which of the following does not have a tetrahedral structure?
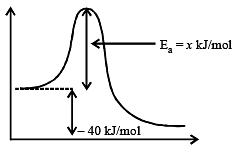
For the equilibrium, ![]() kJ/mol. If the ratio of the activation energies of the forward (Ef) and reverse (Eb) reactions is 2/3 then :
kJ/mol. If the ratio of the activation energies of the forward (Ef) and reverse (Eb) reactions is 2/3 then :
For an octahedral complex, which of the following d-electron configuration will give maximum CFSE?
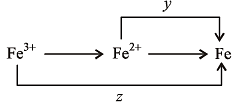
Calculate the standar d cell potential (in V) of the cell in which following reaction takes place : Fe2+ (aq) + Ag+ (aq) → Fe3+ (aq) + Ag(s)
Given that
![]()
Synthesis of ethanal commercially from which of the following reagent is the part of green chemistry ?
The correct order for acid strength of compounds CHºCH2, CH3–C≡CH and CH2=CH2 is as follows:
In a gaseous reversible reaction
N2 (g) + O2 (g) ⇌ 2NO(g)+ heat
if pressure is increased, then the equilibrium constant would be :
Select the correct order of evaporation for water, alcohol, petrol and kerosene oil
100 mL O2 and H2 kept at same temperature and pressure. What is true about their number of molecules
Which one of the following noble gases is not found in the atmosphere?
Which polymer is used for making magnetic recording tapes?
Molecules/ions and their magnetic properties are given below

The correctly matched pairs in the above is:
The vapour pressure of water at 20 °C is 17.5 mm Hg. If 18 g of glucose (C6H12O6) is added to 178.2 g of water at 20 °C, the vapour pressure of the resulting solution will be
Th e factor of D G valu es is impor t an t in metallurgy. The DG values for the following reactions at 800ºC are given as :
S2 (s) + 2O2 (g) → 2SO2 (g) ; ∆G = – 544 kJ
2 Zn (s) + S2 (s) → 2 ZnS(s) ; ∆G = – 293 kJ
2 Zn (s) + O2 (g) → 2ZnO (s) ; ∆G = – 480 kJ
Then ∆G for the reaction :
2 ZnS(s) + 3O2 (g) → 2 ZnO(s)+ 2SO2 (g)
The electron in the hydrogen atom undergoes transition from higher orbitals to orbital of radius 211.6 pm. This transition is associated with :
Which one of the following pairs represents stereoisomerism?
The rate constant for a chemical reaction has units L mol–1s–1, order of the reaction will be