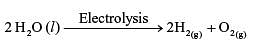Science Olympiad Test: Chemical Reactions and Equations- 2 - Class 10 MCQ
15 Questions MCQ Test - Science Olympiad Test: Chemical Reactions and Equations- 2
Fe2 O3 + 2 Al → Al2O3 + 2 Fe
The above reaction is an example of a
The above reaction is an example of a
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
Which one of the following solution on mixing will not form a precipitate?
Which of the following is (are) on endothermic process(es)?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
When a black and white photographic film is exposed to light, the grey colour on the photographic film is due to presence of
Electrolysis of water is decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is
Green coating on copper in rainy season is due to the formation of
Which of the following substance is reduced in the given reaction below?
PbS(s) + 4H2O2 (aq) → PbSO4(s) + 4H2O
A balanced chemical equation is in accordance with which one of the following laws given below
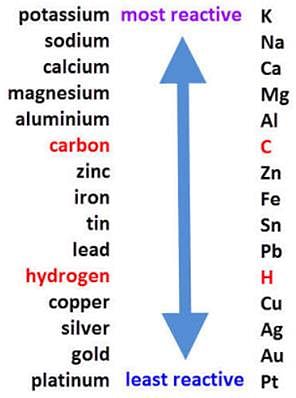
Which one of the following does not result in the evolution of H2 gas?
Which one of the following on mixing with water will result in rise of temperature?
Which one of the following is not a chemical change?
Which among the following is called double displacement reaction (s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2 NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2 O2 → CO2 + 2H2O
Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)