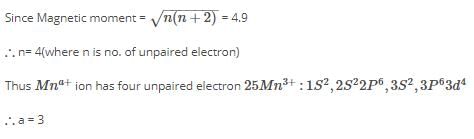Atomic Structure (Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Atomic Structure (Chapter Test - Medical)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If uncertainty in position of electron is zero, uncertainty in its momentum would be
The orbital with maximum number of possible orientations is
According to Bohr’s theory the angular momentum for an electron of 5th orbit is
The ratio of the energy of a photon of 2000 Å wavelength radiation to that of the of 4000 Å radiation is
Photons of energy 6 eV are incidented on a potassium surface of work function 2.1 eV. What is the stopping potential ?
Three isotopes of an element have mass numbers, m, (m + 1) and (m + 2). If the mean mass number is (m + 0.5) then which of the following ratios can be accepted for m, (m +1), (m + 2) in that order ?
An ion Mna+ has the magnetic moment equal to 4.9 BM. The value of ‘a’ is
A photon of wavelength 6630Å is incident on a totally reflecting surface. The momentum delivered by the photon is equal to
Number of protons, neutrons and electrons in the element 89X231 respectively is
If the threshold frequency of a metal for photoelectric effect is v0, then which of the following will not happen?
An orbital with l = 0 is symmetrical round
Which set of quantum numbers given below represents the highest energy of an electron in an orbital ?
The ratio of (E2 – E1) to (E4 – E3) for H–atom is approximately
The number of degenerate orbitals in an energy level of H-atom having En= -RH/9, where RH is Rydberg and 1 RH = 2.18 x 10–18 J is
Which of the following formula involve both wave and particle like behaviour ?
If the energy of an electron in the first Bohr orbit of Hatom is —313.6 kcal/mol; then energy of electron in the second orbit will be
If E1, E2 and E3 represent respectively the kinetic energies of an electron, an α-particles and a proton each having same de Broglie wavelength then