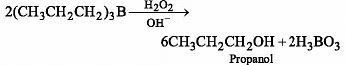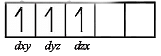NEET Practice Test - 19 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 19
CN– is a strong field ligand. This is due to the fact that
Which property of white phosphorus is common to red phosphorous?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
0.4 moles of HCl and 0.2 moles of CaCl2 were dissolved in water to have 500 mL of solution, the molarity of Cl– ion is:
The decreasing order of stability of alkyl carbonium ion is in the order of:
Which of the following reagents convert propene to 1-propanol?
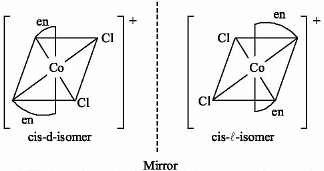
Which one of the following is expected to exhibit optical isomerism?
(en = ethylenediamine)
[Cr(H2O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B. M. The correct distribution of 3d electrons in the Chromium of the complex is
When concentrated HCl is added to an aqueous solution of CoCl2, its colour changes from reddish pink to deep blue. Which complex ion gives blue colour in this reaction?
Hydrogen has an ionisation energy of 1311 kJ mol–1 and for chlorine it is 1256 kJ mol–1.
Hydrogen forms H+ (aq) ions but chlorine does not form Cl+ (aq) ions because.
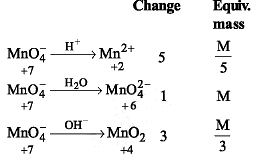
Equivalent weight of KMnO4 acidic medium, neutral medium and concentrated alkaline medium respectively are M/5, M/1, M/3. Reduced products can be
Which of the following statements is not correct for sigma and pi-bonds formed between two carbon atoms?
The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to ratio of the concentrations of the conjugate acid (HIn) and base (In–) forms of the indicator by the expression
An ideal gas expands in volume from 1×10–3 to 1 × 10–2 m3 at 300 K against a constant pressure of 1×105 Nm–2. The work done is
The heats of neutralisation of CH3COOH, HCOOH, HCN and H2S are – 13.2, – 13.4, – 2.9 and – 3.8 kcal per equivalent respectively. Arrange the acids in increasing order of strength
In sodium fusion test of organic compounds, the nitrogen of the organic compound is converted into
Arrange the following elements in the order of ease of detection of wave properties, in the de Broglie experiment.
H, Li, Be, B, K
Aluminium vessels should not be washed with materials containing washing soda since
The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
Which one of the following cannot function as an oxidising agent?
Which of the following compounds has the highest boiling point?
The number of enantiomers of the compound CH3CH(Br)CH(Br)COOH is :
(a)2
(c)4