Solutions (Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Solutions (Chapter Test - Medical)
Semipermeable membrane is that which permits the passage of
Which one of the following solution will have highest osmotic pressure? (assume that all the salts are equally
dissociated)
dissociated)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The diagram given below is a vapour pressurecomposition diagram for a binary solution of A and B.
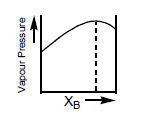
In the solution, A–B interactions are
Which of the following solution will exhibit highest boiling point ?
Two liquids X and Y form an ideal solution. The mixture has a vapour pressure of 400 mm at 300 K when mixed in the molar ratio of 1 : 1 and a vapour pressure of 350 mm when mixed in the molar ratio of 1 : 2 at the same temperature. The vapour pressure of the two pure liquids X and Y, respectively, are
Cryoscopic constant is the depression in freezing point produced by
On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following
cases dissolution of sugar will be most rapid ?
The freezing point order of the solution of glucose is
The depressions in freezing point for 1 M urea, 1 M glucose and 1 M NaCl are in the ratio
Which of the following does not give correct information about osmosis ?
2 moles each of liquids A an B are dissolved to form an ideal solution. What will be the mole fraction of B in the vapour phase?
Consider following cases
I: 2 M CH3COOH solution in benzene at 27°C where there is dimer formation to the extent of 100%
II: 0.5 M KCl aqueous solution at 27°C, which ionises 100% Which is/are true statement(s) ?
Consider the following aqueous solutions and assume 100% ionization in electrolytes
1. 0.1 m urea 2 . 0.04 m Al2(SO4)3
3. 0.05 m CaCl2 4. 0.005 m NaCl
The correct statement regarding the above solutions is
If the vapour pressure of pure A and pure B at 298 K are 60 and 15 torr respectively, what would be the mole
fraction of A in vapour phase(at this temperature) in a solution that contains 20 mole per cent of A in the (A + B) binary mixture in the liquid phase?
Puffiness or swelling , known as edema is due to
If a thin slice of sugar beet is placed in concentrated solution of NaCl then

















