Electrochemistry (Chapter Test - Non-Medical) - Class 12 MCQ
20 Questions MCQ Test - Electrochemistry (Chapter Test - Non-Medical)
Electrolysis of dilute sulphuric acid using inert electrode will produce H2 and O2 in the ratio .... by mass.
What is the potential of the cell containing two hydrogen electrodes as represented below ?
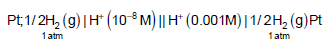
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The rusting of iron takes place as
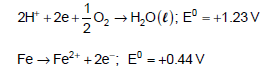
Thus, ΔG0 for the net process is
Solubility of a sparingly soluble salt S, specific conductance K and the equivalent conductance Δ0 are related
as
A galvanic cell is composed of two hydrogen electrodes, one of which is standard one. In which of the following solutions should the other electrode be immersed to get maximum e.m.f. ?
A 250 mL sample of a 0.2 M Cr3+ is elecrolysed with a current of 96.5 amp. If the remaining concentration of
Cr3+ ions is 0.1 M, the duration of electrolysis is
A solution of 50 moles of NaOH in 1 m3 solution offered a resistance of 50 ohm using a cell of cell constant 100 m—1. Molar conductance of the solution is
A 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to
the evolution of chlorine gas at one of the electrodes. The total number of moles of chlorine gas evolved is
The conductivity of 0.25 M solution of a weak electrolyte XY is 0.0125 S cm—1. of the electrolyte
is 500 S cm2 mol—1. The dissociation constant of the electrolyte is
The standard electrode potential of Ag+ / Ag is + 0.80 V and of Cu2+ / Cu is + 0.34 V. These electrodes are
connected through a salt bridge and if
The conductivity of a 0.05 M solution of an electrolysis at 250C is 2 x 10—3 S cm—1. The molar conductance of the solution is (in S cm2 mol—1)
Limiting molar ionic conductivities of A+ and B2— ions are 55 and 75 S m2 mol—1 respectively. What is the molar conductivity of the compound formed between A+ and B2—, at infinite dilution ?
While charging the lead storage battery ........
The overall reaction of a hydrogen-oxygen fuel cell is
Match the column I with column II and mark the appropriate choice.
Q.
Which of the following is most suitable in locating the end point in the above titration ?

















