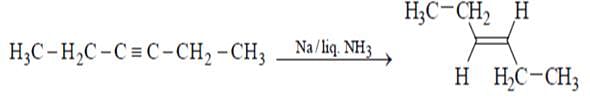Test: Oxidizing and Reducing Reagents - Chemistry MCQ
10 Questions MCQ Test - Test: Oxidizing and Reducing Reagents
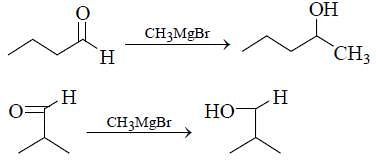
How many isomers of C4H8O when reacts with CH3MgBr followed by acidification to alcohol (only consider carbonyl isomers and Including stereoisomers)?
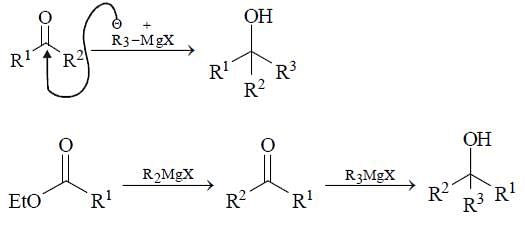
How can we prepare RR’ R” OH by the action of excess of a suitable Grignard reagent on which of the following reactants?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
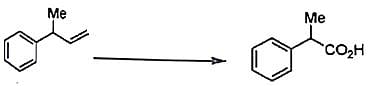
Which is the correct combination of reagent which can carry out following conversion?

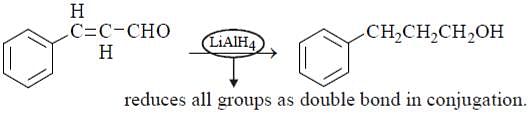
Which is the mildest reducing agent which reduces only carbonyl group in presence of nitro, carboxyl, double bond and ester groups?
Which of the will give effective reduction of 3-hexyne to trans-3-hexene?
What is used to carry out the following conversion?
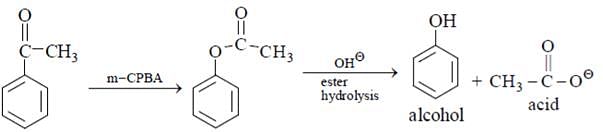
How acetophenone can be converted to phenol by reaction?
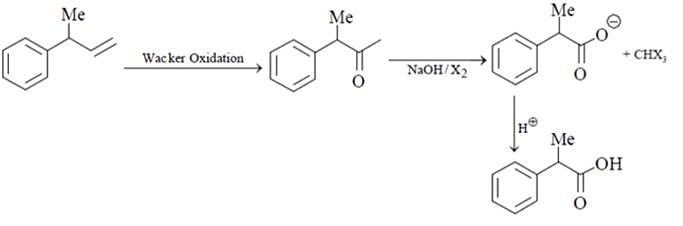
What will be the product for the given reactant and reagents?
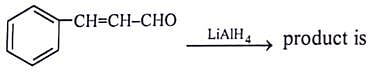
Which of the reagent will give effective transformation of given compounds?

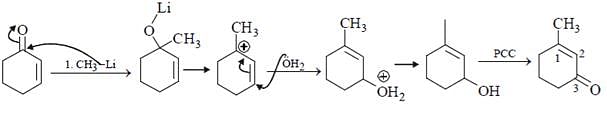
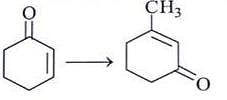
Which is the suitable catalyst for bringing out the transformation given below?
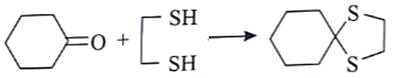


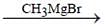 2° Alcohol, if we consider only carbonyl isomer.
2° Alcohol, if we consider only carbonyl isomer.