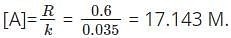Test: Rate of a Chemical Reaction - Chemistry MCQ
10 Questions MCQ Test - Test: Rate of a Chemical Reaction
How many times will the rate of the elementary reaction 3X + Y → X2Y change if the concentration of the substance X is doubled and that of Y is halved?
What is the rate law for acid hydrolysis of an ester such as CH3COOC2H5 in aqueous solution?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The reaction NO2 + CO → NO + CO2 takes place in two steps. Find the rate law.
2NO2 → NO + NO3 (k1) – slow
NO3 + CO → CO2 + NO2 (k2) – fast
2NO2 → NO + NO3 (k1) – slow
NO3 + CO → CO2 + NO2 (k2) – fast
The rate constant of a reaction is k=3.28 × 10-4 s-1. Find the order of the reaction.
The decomposition of NH3 on platinum surface is zero order reaction. If k = 2.5 × 10-4 mol L-1s-1 the rate of production of H2 is
What is the concentration of the reactant in a first order reaction when the rate of the reaction is 0.6 s-1 and the rate constant is 0.035?
For the reaction A + H2O → products, find the rate of the reaction when [A] = 0.75 M, k= 0.02.
For a reaction A +B → C, the experimental rate law is found to be R=k[A]1[B]1/2. Find the rate of the reaction when [A] = 0.5 M, [B] = 0.1 M and k=0.03.
For a second-order reaction, what is the unit of the rate of the reaction?
The rate law for the reaction involved in inversion of cane sugar is R=k [C12H22O11] [H2O].