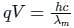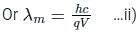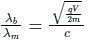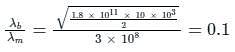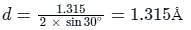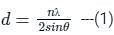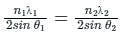Test: Bragg’s Law - Chemistry MCQ
10 Questions MCQ Test - Test: Bragg’s Law
In the X-ray diffraction of a set of crystal planes having d equal to 0.18 nm, the first-order reflection is found to be at an angle of 22°. The wavelength of X-rays is: (sin 22° = 0.208)
If an X-ray tube operates at the voltage of 10 kV, find the ratio of the de-Broglie wavelength of the incident electrons to the shortest wavelength of X-rays produced. The specific charge of an electron is 1.8 × 1011 C / kg.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the X-ray diffraction of a set of crystal planes having d equal to 0.18 nm, first order reflection is found to be at an angle of 22°. The wavelength of X-rays is: (sin 22° = 0.374, cos 22° = 0.927)
The diffraction pattern of copper metal was measured with X-ray radiation of wavelength of 1.315 Å. The first order Bragg diffraction peak was found at an angle 2θ of 60°. The d-spacing between the diffracting planes in the copper metal is
A beam of X-rays is constructively scattered in second order from the surface of the crystal at an angle of 30° and the spacing between layers of atoms in NACl crystal is 4.5 × 10-10 m. The wavelength of X-rays is ______
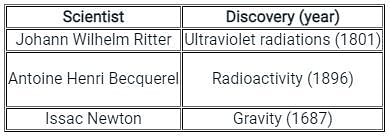
X-ray crystallography is not used to find the physical properties of which of the following?
Two lines, A and B, of an X-ray beam, give second-order reflection maximum at a glancing angle of 60° and third-order reflection maximum at an angle of 30° respectively from the face of the same crystal. If the wavelength of line B is 0.25 nm, then the wavelength of line A will be:


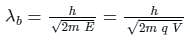
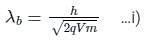
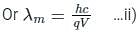 ngth of X-rays, we have
ngth of X-rays, we have