Test: Stoichiometry - JAMB MCQ
10 Questions MCQ Test - Test: Stoichiometry
Which of the following is not true regarding balanced chemical equations?
What is the amount of water produced when 8g of hydrogen is reacted with 32g of oxygen?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
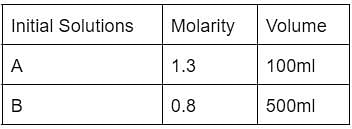
A and B are two solutions that are mixed. Calculate the resultant solution’s molarity.

In a container, there are 4 moles of nitrogen, 3 moles of oxygen and 7 moles of hydrogen; find out the mole fraction of oxygen in this reaction.
In a particular reaction, one of the reactants limits the number of products formed. That is called as _______
Which of the given reactions are counted as balanced reactions?
Calculate the mass percent of magnesium in the formation of magnesium oxide.
A solution contains 8 moles of solute and the mass of the solution is 4 kg. What’s the molality of this solution?
Find the amount of carbon dioxide produced by the combustion of 20g of methane.

















