Test: Soil Structure & Clay Mineralogy - Civil Engineering (CE) MCQ
10 Questions MCQ Test - Test: Soil Structure & Clay Mineralogy
The octahedral structural unit of clay minerals consists of _______ hydroxyls forming a configuration of an octahedron and having one aluminium atom at the centre.
Expansive soils are those which generally consists of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Select the sequence of clay minerals in decreasing order with respect to the plasticity index is
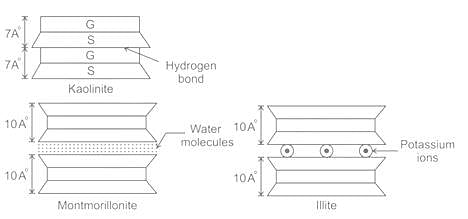
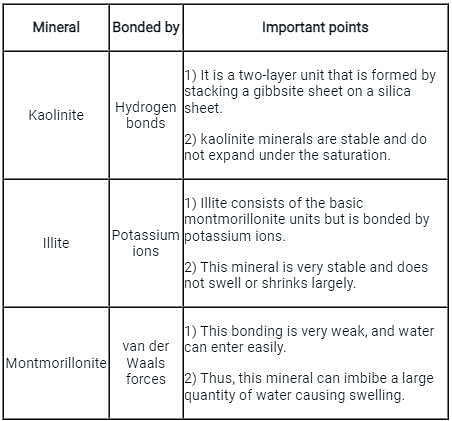
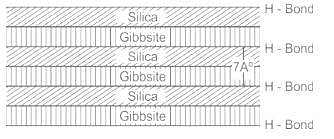
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?
Which kind of structure is formed if the net electrical forces between adjacent soil particles at the time of deposition are repulsive?
Which of the following clay mineral present in black cotton soil is a cause for its large swelling and shrinkage?
Clay minerals in soils are produced due to ___________ type of weathering.
The thickness of diffuse double layer in pure clay will be maximum for the following predominant clay mineral: