Chemical Equilibrium ( Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Chemical Equilibrium ( Chapter Test - Medical)
For the equilibrium AB(g) ⇔ A(g) + B(g) at a given temperature, the pressure at which 50% of AB is
dissociated is numerically equal to
dissociated is numerically equal to
For the equilibrium MgCO3(s) ⇔ MgO(s) + CO2(g), which of the following expression is correct ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the reaction 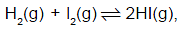 value of the equilibrium constant depends on
value of the equilibrium constant depends on
The conditions which favour the manufacture of ammonia by the reaction (Le-Chatelier’s principle)
1 mole of H2 and 2 moles of I2 are taken initially in a 5 litre vessel. If the number of moles of H2 at equilibrium is 0.2, the number of moles of I2 and HI, respectively at equilibrium would be
Consider the reaction 2H2 + O2  2H2O.
2H2O.
Which of the following correctly states the expression for the equilibrium constant for this reaction?
1.2 moles each of pure acetic acid and 1.2 moles of pure ethanol are subjected to esterification. At
equilibrium 0.4 moles of ester were found to have formed. The equilibrium constant for the reaction
At a rather high temperature the Kp values are computed as
What is the Kp for the reaction ?
Kc for a reaction A + 2B ⇔ D, is 0.64. What should be the concentration of B when it is in equilibrium with
equimolar quantities of A and D ?
In which of the following, Kp is not greater than Kc?
Given the equation,
Which combination of pressure and temperature should give the best yield of Z ?
The equilibrium constant Kp for the reaction
is 16. If the volume of the container is reduced to one half of its original volume,the value of Kp for the reaction at the same temperature will be
The equilibrium constant for the given reaction is 100.
What is the equilibrium constant for the reaction given below
For the reaction,
the formation of Cl(g) is favoured at
In the system
doubling the mass of CaO will cause the equilibrium concentration of CO2 to
In equilibrium constant expression, concentration of products is taken in/on the
Given the equilibrium reaction,
This reaction is studied by starting with equal amounts of A and B in a constant-volume vessel. Which of the following expressions will hold good ?
In a system
if the equilibrium concentration of B increases to 8 times, it will cause the equilibrium concentration of C to change to
For the heterogeneous equilibrium
the temperature is given by
Given
The equilibrium constant K4 for the following reaction
may be given as

















