Chemical Equilibrium ( Chapter Test- Non Medical) - Class 12 MCQ
20 Questions MCQ Test - Chemical Equilibrium ( Chapter Test- Non Medical)
For a reaction,
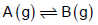
at equilibrium the partial pressure of B is found to be one fourth of the partial pressure of A. The value of ΔG0 of the A →B reaction is
For an equilibrium reaction, which one of the following
is not true ?
is not true ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For the reaction

the relation connecting the degree of dissociation (a) of N2O4(g) with the equilibrium constant Kp and (p)
equilibrium pressure is
equilibrium pressure is
The equilibrium constant of the reaction,
is 1.60. If 0.4 mol of each is H2 and CO2 are enclosed in
a 1.0 litre vessel, the equilibrium concentration of H2O(g) will be
Consider the reactions
The addition of an inert gas at constant volume
Acetic acid undergoes dimerisation, when dissolved in benzene
Molecular mass of acetic acid is found 120. Which among the following relation is correct ?
Ka for the acid HA is 1 x 10–6. The value of K for the reaction,
For an ideal-gas reaction, 2A + B ⇔C +D , the value of Kp will be
For the reaction,
AB is 33% dissociated at a total pressure of P. Therefore, P is related to Kp by one of the following options
Partial pressure of O2 in the reaction
is
Consider the reaction
What will be the concentration of HI at equilibrium if one mole each of H2,I2 and HI are together taken in one litre flask and allowed to react ?
Equilibrium is likely to shift from left to the right of a chemical equation A ⇔ B when
3.1 mole of FeCl3 and 3.2 mole of NH4SCN are added to one litre of water. At equilibrium, 3.0 mol of FeSCN2+ are formed. The equilibrium constant Kc of the reaction
will be
For the reaction,
Kp = 63 atm at 100 K. If at equilibrium, , then the total pressure of the gases att equilibrium is
For a physical equilibrium which of the following is true ?
The Kp/Kc ratio for the reaction
at 1270C is
For the equilibrium
Kc = 1.0 x 10–5. If the equilibrium concentration of H2 and Cl2 is 1.2 x 10–3 M and 1.2 x 10–4 M respectively then the concentration of HCl is
When 20 g of CaCO3 is put into a 10 L flask and heated to 1000 K, 40% of CaCO3 remains undissociated at equilibrium. Calculate the value of Kp for the reaction
Ammonium carbamate when heated to 2000C gives a mixture of NH3 and CO2 vapours with a density of 16.0. What is the degree of dissociation of ammonium carbamate ?
The value of ΔU0 for the reaction
for which and
T = 300 K, is

















