BITSAT Chemistry Test - 2 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 2
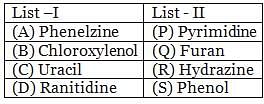
The correct match between items of List-I and List-II is:

The binding of oxygen by haemoglobin (Hb) forming (HbO2), is partially regulated by the concentration of H3O + and dissolved CO2 in blood.
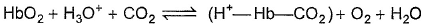
Release of O2 is favoured when there is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Aerated water contains CO2 dissolved in water
CO2(g) + H2O(l) H2CO3(aq)
H2CO3(aq)
Variation of solubility (s) with pressure (p) is shown by
 H2CO3(aq)
H2CO3(aq)Variation of solubility (s) with pressure (p) is shown by
If pKb for fluoride ion at 25°C is 10.83, the ionisation constant of hydrofluoric acid in water at this temperature is :
The pH of an aqueous solution of 1.0 M solution of a weak monoprotic acid which is 1% ionised is:
In which of the following set of compounds oxidation number of oxygen is not (- 2)?
Which pair is not in the correct order of lattice energy?
For the process, and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. For an ideal gas, consider only (p -V) work in going from initial state X to the final state Z. The final state Z can be reached either of the two paths shown in the figure. Which of the following choice (s) is (are) correct?
(Take ΔS as change in entropy and W as work done)
[IIT JEE 2012]
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data etc. Two questions related to the paragraph have been given. Each has only one correct answer among the four given options (a) ,(b), (c) and (d).
Passage
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.
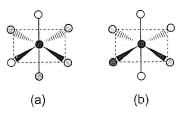
Q.
The oxidation state and coordination number of cobalt in the complex [Co(NH3)4Br2]+ are
Passage
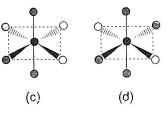
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.


Q.
Which of the structures is identical?
Which of the following are the examples of strong nucleophiles but weak base in protic solvents?
I. CH3S-
II. CH3O-
III. I-
IV. H2O
V. F-
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
An alcohol (R — OH) can be converted into alkyl chloride by the treatm ent with HCI. Reaction involves protonation of alcohol followed by the formation of carbocation intermediate. Carbocation intermediate in the final step undergo nucleophilic attack by Cl- ion as :
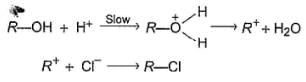
Q.
Which of the following alcohol reacts most easily?
An alcohol (R — OH) can be converted into alkyl chloride by the treatm ent with HCI. Reaction involves protonation of alcohol followed by the formation of carbocation intermediate. Carbocation intermediate in the final step undergo nucleophilic attack by Cl- ion as :
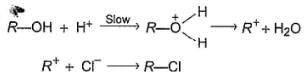
Q.
Which of the following can catalyse the above reaction?
Which of the following is not a bactericidal Antibiotic:
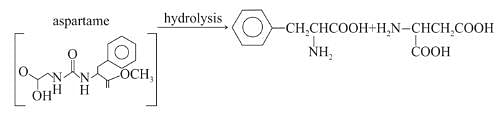
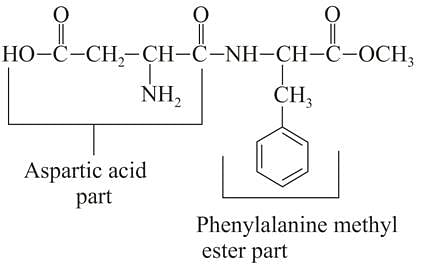
Assuming that both, amide and ester bonds of Aspartame are hydrolysed in the stomach, the amino acids obtained is:
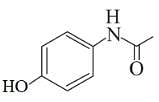
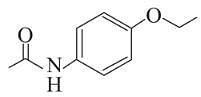
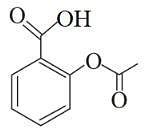
Following are the structures of three drugs A, B and C , respectively,
(a) 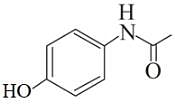
(b) 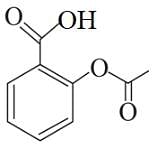
(c) 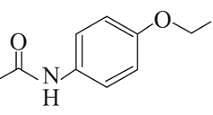
Which of the following drug is capable of destroying microorganisms?
What are lysergic acid diethylamide (LSD) and mescaline?
The substances which do not act as antiseptic are:
(a) Iodoform
(b) H2O2
(c) Diphenhydramine
(d) Soframycin
(e) Amoxycillin
(f) Ranitidine
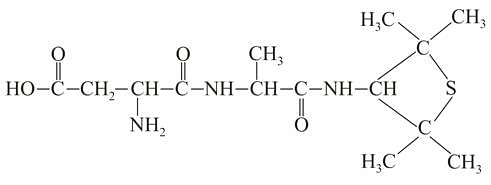
A chemist has 4 samples of artificial sweetener A , B , C and D . To identify these samples, he performed certain experiments and noted the following observations:
(i) A and D both form blue-violet colour with ninhydrin.
(ii) Lassaigne extract of C gives positive AgNO3 test and negative Fe4[Fe(CN)6]3 test.
(iii) Lassaigne extract of B and D gives positive sodium nitroprusside test. Based on these observations which option is correct?