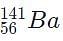Test: Atomic Nucleus - MCAT MCQ
10 Questions MCQ Test - Test: Atomic Nucleus
Which of the following will be a decay product when 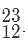 M, g undergoes beta minus decay?
M, g undergoes beta minus decay?
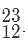 M, g undergoes beta minus decay?
M, g undergoes beta minus decay?When a nucleus undergoes ordinary fission into two daughter nuclei, what happens to the binding energy of the parent nucleus?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Suppose that natural nitrogen is found in 70% abundance as the isotope 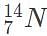 and the rest of the time as
and the rest of the time as 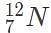 . In this scenario, which of the following would give the atomic mass of this element?
. In this scenario, which of the following would give the atomic mass of this element?
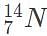 and the rest of the time as
and the rest of the time as 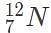 . In this scenario, which of the following would give the atomic mass of this element?
. In this scenario, which of the following would give the atomic mass of this element?The amount of mass of a highly unstable isotope versus time in shown in the accompanying plot. Which of the following is the best estimate of the amount of the half-life of the isotope?
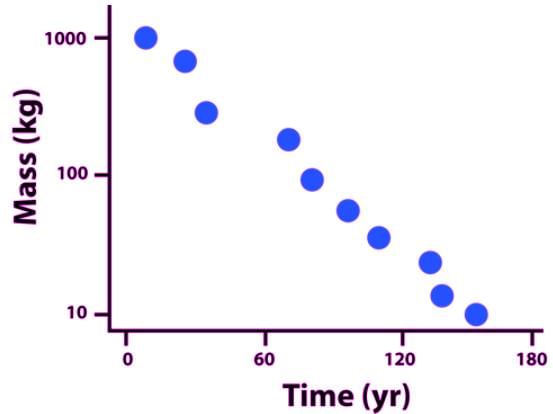
Suppose 64 atoms of a highly unstable Polonium isotope (half-life 10 seconds) are held in a closed container. Which of the following provides the best estimate of when there will no longer be any of the Polonium isotope left in the container?
Which of the following pairs of charged isotopes would be impossible to distinguish using a standard mass spectrometer?
Suppose that, in a mass spectrometer, charged isotopes enter the device with velocities along a direction that is neither perpendicular nor parallel to the magnetic field lines. Which of the following behaviors would result?
If there is 10 kg of a radioactive isotope with a decay rate of 0.1 1/s, how much of the isotope will be left in 30 s?
Which of the following isotopes is the most likely X in the reaction 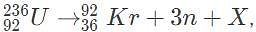 where n denotes a free neutron?
where n denotes a free neutron?
Which of the following decay processes results in the largest change in mass of a nucleus?


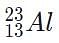 .
.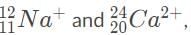 are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.
are thus impossible to distinguish because they have the same charge-to-mass ratio and thus are deflected the same amount by the magnetic field.